Xanthophyll is a type of accessory pigment or phytochemicals, which belongs to the class of “Carotenoids”. In many vascular plants and algae, xanthophylls act as the light-harvesting protein complexes. Xanthophylls are rich in antioxidants, which prevent the cells from damaging. In photosynthetic eukaryotes, the xanthophylls are usually bound to the chlorophyll molecules.
Xanthophylls are the pigment molecules present within the light-harvesting complex, which protect the photosynthetic organisms from the toxic effect of light. In this context, you will get to know the meaning, molecular structure, occurrence, types, cycle, functions and isolation of xanthophyll.
Content: Xanthophyll
- Meaning of Xanthophyll
- Molecular Structure
- Occurrence
- Types
- Xanthophyll Cycle
- Functions
- Isolation of Xanthophyll
Meaning of Xanthophyll
Xanthophyll merely refers to the light-harvesting accessory pigments, which work coordinately with the chlorophyll-a. It can absorb light of a wavelength in a range of 425-475 nm. Xanthophylls are primarily of three types, namely lutein, zeaxanthin and cryptoxanthin. They are highly antioxygenic molecules, which protect the cell from damage and ageing.
Xanthophyll is highly beneficial for eye health, as it reduces the risk of eye cataract and macular degeneration. Xanthophylls or Phylloxanthins are the yellow colour pigment naturally present in the plants. A xanthophyll can isolate from the plant extract.
You can isolate the xanthophyll pigment from the plant extract by performing chromatography, which results in the formation of a yellow colour band over a chromatography paper. Let us look into some of the general properties of the xanthophyll.
| Properties | Xanthophyll |
|---|---|
| Molecular formula | C40H56O2 |
| Molecular weight | 568.886 g/mol |
| Charge | Neutral |
| Physical state | Liquid |
| Colour | Yellow |
| Nature | Polar compound |
Molecular Structure
Xanthophyll is the primary accessory pigment. It consists of C-40 terpenoid compounds, which forms as a result of condensation between the isoprene units. The molecular structure of xanthophyll and carotene (another accessory pigment) is almost the same except for the presence of an oxygen atom. In xanthophyll, there is an oxygen atom present as the hydroxyl group, whereas carotene lacks an oxygen atom and exists as a pure hydrocarbon.

Occurrence
Xanthophylls occur naturally in the plants, which regulate the light energy and act as “Photochemical quenching agent” that deals with the exciting form of chlorophyll or triplet chlorophyll. The triplet chlorophyll produces at a higher rate during the photosynthetic process. Xanthophylls are also found in the body of humans and animals, which comes ultimately by the source of green plants.
Types
Xanthophylls mainly include accessory pigments like lutein, Zeaxanthin and cryptoxanthin.
- Lutein: It is the most common xanthophyll, which is synthesized by the green plants itself. Spinach, kale, kiwi, green apples, egg yolk, corn etc. are the sources of lutein. Lutein is a lipophilic molecule (insoluble in polar solvent like water). In plants, lutein is present as fatty acid esters, in which one or two fatty acids attach with the two –OH groups. Lutein mainly absorbs blue light, and thereby it protects the eye from the blue light that leads to an eye impairment.
- Zeaxanthin: It merely refers to the carotenoids alcohols, which can be synthesized naturally by the plants and certain microorganisms. It acts as a non-photochemical quenching agent. Zeaxanthin is an accessory pigment, which gives distinct colour to the corn, wolfberries etc. It consists of two chiral centres. Kale, spinach, turnip greens, mustard greens etc. are the sources.
- Cryptoxanthin: Its molecular structure is quite similar to the β-carotene, but a hydroxyl group is present in addition. Cryptoxanthin is found as red crystalline solid in its pure form. It also refers to provitamin-A, as during the xanthophyll cycle, cryptoxanthin converts into vitamin A (retinol).
Xanthophyll Cycle
The xanthophyll cycle occurs inside the thylakoid membrane of the chloroplast. Xanthophyll cycle facilitates the interconversion of oxygenated carotenoids. There are many types of xanthophyll cycle, but violaxanthin and Diadino xanthin cycle are the most common.
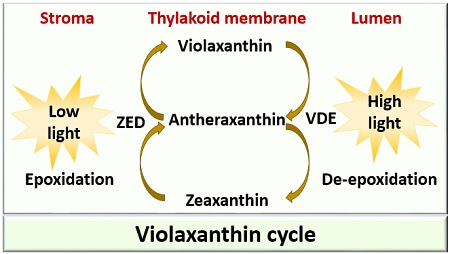
Violaxanthin Cycle
Firstly, violaxanthin (has two epoxide group) converts into antheraxanthol (has one epoxide group). Antheraxanthol further turns into Zeaxanthin (has no epoxide group). VDE, i.e. violaxanthin de-epoxidase catalyses the conversion of violaxanthin to zeaxanthin as a result of the de-epoxidation reaction.
ZE, i.e. zeaxanthin epoxidase is an enzyme, which catalyses the conversion of zeaxanthin into violaxanthin as a result of epoxidation reaction. The epoxidation reaction will occur at a low pH <5.8 under low light, whereas de-epoxidation reaction occurs at a high pH of 7.5 under a high light source.
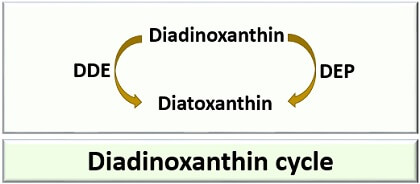
Diadinoxanthin Cycle
The diadinoxanthin cycle is another type of xanthophyll cycle commonly found in diatoms and eukaryotic algae. It is a reversible diadinoxanthin cycle, which can covert diadinoxanthin into diatoxanthin and vice versa. DEP, i.e. diadinoxanthin epoxidase catalyses the conversion of diadinoxanthin into diatoxanthin. DDE, i.e. diatoxanthin de-epoxidase, catalyses the conversion of diatoxanthin into diadinoxanthin. Some algae make the use of both violaxanthin and diadinoxanthin cycle.
Functions
Xanthophylls perform two central roles:
- Harvesting of light: Xanthophylls are the accessory pigments, which act as a photosynthetic light-harvesting complex of algae and vascular plants.
- Dissipation of energy as heat: Xanthophyll helps in photoprotection, i.e. it protects the photosynthetic apparatus from the photo-oxidative damage in the condition of excessive light by dissipating energy.
Isolation of Xanthophyll
A xanthophyll can be isolated by the method of chromatography. For this, prepare a plant extract by crushing the fresh leaves. Place a drop of leaf extract at one end (above 1 cm) on chromatography paper (considered as a stationary phase). Then, take acetone ligroin mixture as a non-polar hydrophobic solvent (considered as a mobile phase), which will run through the filter paper.
Add solvent and hang the filter paper inside the chromatography chamber. Cover the chromatography chamber with a lid to prevent gas exchange. As the solvent mixture comes in contact with the leaf extract, it will help in the migration of the different plant pigments at a different rate.
The separation and the travelling distance of plant pigment are based on their solubility with the solvent used. The different plant pigments like chlorophyll, xanthophyll and carotene will travel at different rates and appear as different bands. Chlorophyll being highly polar, it will adhere to the polar surface of the paper.
Thus, chlorophyll moves the shortest distance and appears as “Green band”. Xanthophyll being less polar will move a shorter distance and appear as “Yellow band”. Carotene being non-polar will attract more strongly to the non-polar solvent and move along with it. Thus, carotene will move the longest distance and appear as “Orange band”.
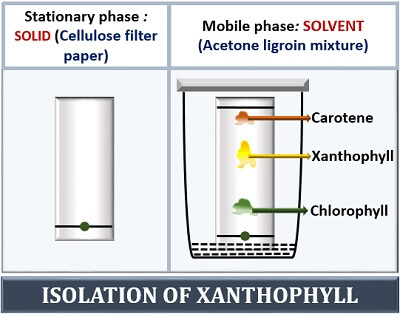
The solvent is allowed to run to the distance (by leaving 1 cm distance from the top) and called a “Solvent front”. The formation of different bands on the chromatography paper commonly refers to the “Chromatogram”. The Rf value is calculated by the ratio of distance travelled by the pigment, and the total distance travelled by a solvent.
I liked the article.