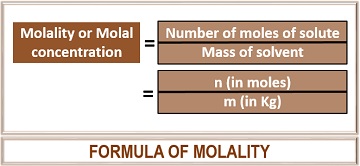
Molality is a physical quantity that measures the concentration of solute (expressed as the number of moles) dissolved per kilogram of solvent. Thus, molality can calculate the mass of both the solvent and the solute. It also refers as “Molal concentration”.
It can be calculated by dividing the number of moles of solute by the mass of solvent in kilograms. The S.I unit of molality represents as ‘mol/kilogram’ or ‘m’. Thus, molality is another way to calculate the concentration of a solution.
People are usually confused with the term molarity. The difference between molality and molarity is in the denominator, i.e. between the solvent and the solution. Therefore, molality is related to the mass of the solvent, not the solution.
Content: Molality
Definition of Molality
Molality can be defined as the molal concentration of the solution, which contains 1 mole of solute dissolved in one kilogram of solvent and express as mole/kilogram. It denotes the lower case ‘m’ and is written as m or -m. A solute is a substance that is being dissolved in the solvent, whereas a solvent dissolves the solute added into it. Therefore, both solute and solvent combine to form a solution. Thus, molal concentration can be determined by knowing the mass of solute and the mass of solvent.
How to Calculate
Let us study some conditions to calculate molality and its relative quantities.
A. If we have to find out molality, then we can put the values in the given formula:
Molality (m) = number of moles of solute / Mass of solvent in Kg
B. If we have to find out the moles of solute, then we can compute the equation as:
Number of moles of solute = m X Mass of solvent
C. Similarly, if we have to find out the volume of the solution, then the equation can express as:
Mass of solvent = number of moles of solute / m
So, these are the basic formulas or equations through which we can calculate the molality, mass of solute or the mass of solvent as well.
D. If the value of solute and solvent is given in grams, then the formula is represented as:
m = nsolute / msolvent
m = Gram weight of solute / Molecular weight of solute ÷ Weight of solvent / 1000
m = Gram weight of solute / Molecular weight X 1000 / Weight of solvent
E. Sometimes, the value of solvent is given in density (g/cm3), so to convert it into kg, we have to put the formula of density first:
Density = Mass / Volume
Mass = Density X volume
The value of mass obtained will be the mass of the solution. Thus the mass of solvent can be obtained by the formula:
Mass of solvent = Mass of the solution – Mass of solute
Simple Calculations
Let us take an example to understand the concept of molality more briefly, by a question given below:
Ques. Find out the molality of a solution containing 30 grams of NaCl in 15 g of water?
To calculate molality, look at the parameters given in the question:
Case-1: First, find out the moles of solutes dissolved in a solvent. According to a question, 30 grams of solute (NaCl) is present. In molality, the value of solute always expresses in moles. Thus the value of 30 grams of NaCl should convert into moles.
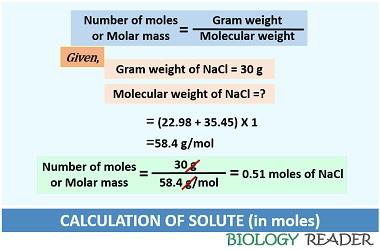
Number of moles of solute = 30 g NaCl / 58.4 g per mol NaCl = 0.51 mol of NaCl
Case-2: Second, find out the mass of the solvent. According to a question, 15 g of solvent is present in a solution. In molality, the value of the solvent always expresses in kilograms. Thus the value 15g should convert into a kilogram.
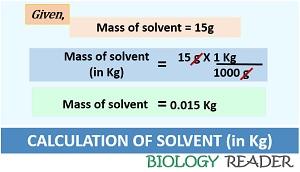
Mass of solvent (in Kg) = 15g / 1000 = 0.015 Kg
Case-3: Third, find out the molality once you have calculated the number of moles of solute and the mass of solvent. Thus, the value of the molarity can calculate as:
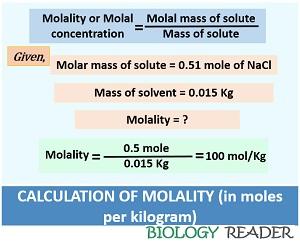
m (mol/Kg) = 0.51 mol/ 0.015 Kg = 100 mol/Kg
Advantages
If a solution is diluted nearly to 1.0g/mol at room temperature, the values of both molality and molarity are the same, but when the concentration of solution changes, the values of both quantities change.
The calculation of molality involves the measurement of solute and solvent mass that does not change by altering temperature. In contrast, molarity deals with the mass of solute in the volume of solution that changes with the altering temperature and pressure.
Thus, molality has an advantage over the molarity by the following means:
It deals with the measurement of the mass of solute and solvent, whose value is important to know the chemical composition. The mass remains unchanged under the effect of temperature and pressure, whereas the volume of a solution changes. The value of molality can easily convert into a mass ratio or mole fraction.