Regeneration in plants can define as a standard method in the field of biotechnology, where the small section of tissue or explant can develop into a complete plant under in-vitro conditions. It also depends upon the concentration of plant regulatory hormones like auxin, cytokinin, gibberellins etc. where some may influence, or others may inhibit the growth.
A plant consists of totipotent cells that help in regenerating new cells. Thus, the totipotency of plant can define as the characteristic property of the plant cells which are having the potential to retain the confined damage in the injured tissues or cells, and having the ability to develop a complete plant.
Content: Regeneration in Plants
Definition of Regeneration
Regeneration is a biological process in which new organized structures like root, shoot, buds etc. can regenerate by the culturing of tissue section under controlled conditions. This technique mainly includes two methods, namely organogenesis and embryogenesis, which can be accomplished directly by taking the explant or subjecting to the callus phase. A term regeneration is sometimes interchangeable with the term “Differentiation”.
Thus, sometimes root and shoot-regeneration also refers to as root and shoot differentiation. Root differentiation is only useful in conjugation with a shoot and embryo germination, whereas shoot germination is much effective method for the growth of a fully organized plant. The regeneration of plant organs depends upon the cellular differentiation of callus tissue.
Regeneration or “Totipotency” can be studied in vitro by culturing the explant in a nutrient medium added with some growth regulating phytohormones. There are two standard cellular strategies for the technique of regeneration. The first strategy is the reactivation of undifferentiated cells and the second strategy is the reprogramming of the differentiated somatic cells. The process of renewal occurs naturally starting from the cut-sites.
Methods of Regeneration in Plants
In Plants, the totipotent cells differentiate into new tissues or organ commonly by the two ways:
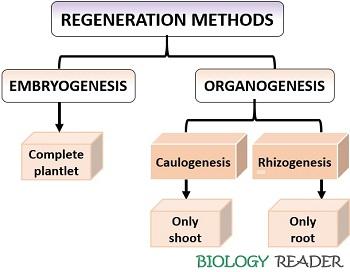
Organogenesis
It is a popular method in the production of the new adventitious shoot and root system either from an explant or from the undifferentiated cellular mass (callus). The auxin and cytokinin ratio governs the shoot and root regeneration. The tissue in the culture medium will respond to the phytohormones. Generally, there are three stages of organogenesis like competence, differentiation and morphogenesis.
By the method of explant culturing, shoots or buds will develop directly, while shoots or roots can be produced indirectly from an intervening callus phase. The technique of forming adventitious shoot bud refers as “Caulogenesis” while the process of forming adventitious roots refers as “Rhizogenesis”.
Sometimes organoids develop from the peripheral region of the callus tissue, not from the meristemoids as a result of error while performing the experiment. Organoids can define as the anomalous structures, which differ from the actual organ or tissue. In contrast, the localized meristematic cells of the callus can define as meristemoids that can develop shoots and roots.
Shoot Regeneration or Caulogenesis
It defines as the process of growing adventitious shoot bud from the meristemoids, which eventually produce leaf primordia and apical meristem. The bud will develop procambial strands connected with the pre-existing vascular tissue in the callus. Then transfer the callus to the appropriate culture medium, clusters of meristematic cells or nodules will appear.
The nodules will appear in the regions accumulated with the starch that provides the energy source for the shoot bud differentiation. Gibberellic acid is a phytohormone that impedes the accumulation of starch and thus inhibits the differentiation of shoot bud. Meristemoids develop vascular elements inside and cambium like cells outside them.
Eventually, the meristemoids will produce either root endogenously or shoot exogenously. In a few cases, the shoots also develop endogenously. Auxin to cytokinin ratio, the genotype of explant, the physical condition of the medium, environmental conditions etc. influence the shoot differentiation.
Root Regeneration or Rhizogenesis
This technique is not much used in the viewpoint of plant transformation studies. In Rhizogenesis, root tip of either primary or lateral roots is taken into the ordinary media. As a result of this process, the growth is potentially unlimited.
Somatic Embryogenesis
The in-vitro culturing of an embryo derived from the somatic cell refers as “Somatic embryogenesis”. It also involves direct isolation from an explant and indirect method via callus formation, and basically includes the following steps:
- Initiation of embryonic culture
- The proliferation of embryonic culture
- Prematuration of somatic embryos
- Maturation of somatic embryos
- Plant development on non-specific media
The first two steps require an auxin-rich medium, where the explant cells dedifferentiate to grow into the localized meristematic cells or embryonic clumps. Then transfer the meristematic cells from the induction medium to the standard medium containing low or no auxin, to develop mature embryos.
After that, transfer the somatic embryos to the medium rich-in glucose and having an appropriate concentration of abscisic acid followed by desiccation to produce a more sturdy and hardy somatic embryo. It results in the germination of the somatic embryo when it gets mature enough to possess functional root and shoot apices.
Developmental Pattern
The following steps can depict the developmental pattern of somatic embryos:
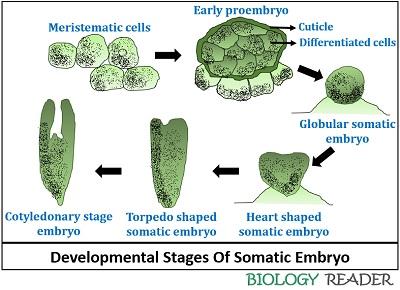
- First, a somatic embryo originates from a single cell that further undergoes cell-division to forms a group of meristematic cells.
- The meristematic cells divide to form an undifferentiated cellular mass refers as “Callus” by the cytoplasmic division between the neighbouring cells.
- Around the differentiated cellular mass, cutinized wall forms.
- The somatic proembryo differentiates into globular somatic embryos, and later into heart-shaped, torpedo-shaped and cotyledonary stage somatic embryos.
- After that, the phase of somatic embryo conversion occurs where the somatic embryo begins to germinate after the cotyledonary embryonic stage.
Sometimes, somatic embryo shows abnormal developmental features like three or more cotyledons, bell-shaped cotyledons etc. These abnormal features can prevail by the addition of abscisic acid or mannitol. Somatic embryos regenerating from explant or intervening callus phase termed as “Primary somatic embryos”.
In many cases, secondary somatic embryos develop from the tissue of somatic embryos or the parts of germinating somatic embryos. The SEs regenerate from the epidermal cells of explant or callus. The somatic embryos are bipolar, possessing both radicle and plumule. The radicular end evolves from the centre of callus, while the plumular end stands out of the callus.
Factors Influencing
There are three significant factors influencing somatic embryogenesis, namely phytohormones, nitrogen source, explant, genotype etc. Auxin (2, 4-D) is a phytohormone that is required by most of the species undergoing somatic embryogenesis. Nitrogen source is another factor showing a marked effect on somatic embryogenesis.
Low NH4+ concentration in combination with NO3– provides a sole nitrogen source that helps in induction, development of the somatic embryos. The genotype of explant is a factor which determines whether SE regeneration will occur or not. Other factors like high K+ and low O2 level promote SE regeneration in a few species.