Molarity is a quantitative measure to determine the concentration of solute (expressed as the number of moles) dissolved in per litre of solution. It also refers to the molar concentration of a solution. Thus, molarity is a quantity which calculates the volume of the solvent or the amount of solute. The concentration of solute can define as the relative amount of solute present in the solution and depends upon the dilution rate of solute in a solution. The strength or concentration of the solvent can define as the volume of solvent (usually water) added to dissolve the solute.
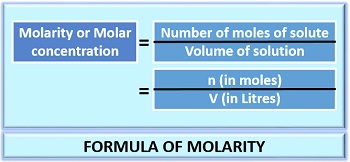
Thus, the molar concentration can obtain by dividing the moles of solute by the total volume. The S.I unit of molarity is represented as ‘mol/litre’ or ‘M’.
Content: Molarity
Definition of Molarity
Molarity can define as the molar concentration of a solute present in one litre of solution, which can express as mole/Litre. The unit of molar concentration is represented by ‘M’.
A solute is a substance like sodium hydroxide (NaOH) that is being dissolved in the solvent. A solvent is usually water which dissolves the solute added into it. Therefore, both solute and solvent combine to form a solution. Thus, molarity can measure the concentration of a solution by giving us the amount of solute and solvent present in the solution.
How to Calculate
To calculate molarity first, we have to find out the given things in a question.
If we have to find out molarity, then we can put the values in the given formula:
- Molarity = number of moles of solute/volume of solution
If we have to find out the moles of solute, then we can compute the equation as:
- Number of moles of solute = Molarity X Volume of solution
Similarly, if we have to find out the volume of the solution, then the equation can express as:
- The volume of solution = number of moles of solute / Molarity
So, these are the basic formulas or equations through which we can calculate the molarity, molar mass of solute or volume as well.
Simple Calculations
Let us take an example to under the concept of molarity more briefly, by a question given below:
Question: Find out the molar concentration of a solution containing 20 grams of NaOH in a 120 ml volume of water.
To calculate molar concentration, look at the quantities given in the question:
Case-1: First, find out the number of moles dissolved in a solution. According to a question, 20 grams of NaOH are present in a solution. In molar concentration, the solute is always expressed in moles. Thus the value 20 grams of NaOH should be converted into moles.
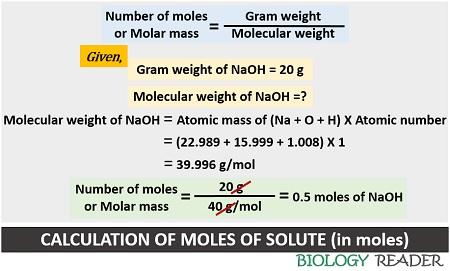
Number of moles of solute = 20 g NaOH X 1 mol NaOH / 40 g NaOH = 0.5 mol of NaOH
Case-2: Second, find out the volume of a solution. According to a question, 120 ml of solvent is present in a solution. In molar concentration, the value of the solvent is always expressed in a litre. Thus, the value of 120 ml should convert into litre.
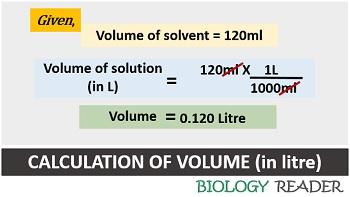
Volume of solution (in L) = 120 ml X 1 L/ 1000 ml = 0.120 L
Case-3: Third, find out the molarity once you have calculated the number of moles of solute and the volume of solution. Thus, we can calculate the value, by the formula expressed as:
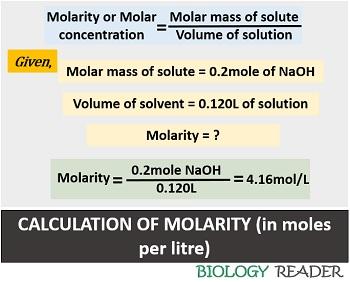
M = n / V
M = 0.5 mol / 0.120 L = 4.16 mol/L