Haemoglobin or Hb is an iron transport metalloprotein. It refers as “Metalloprotein” as it contains four iron metal ions or haem (Iron-containing porphyrin) groups and globular protein. Haemoglobin appears red coloured. Almost all vertebrates and tissues of a few invertebrates contain haemoglobin in their RBCs.
Other than RBCs, haemoglobin is also present in macrophages, alveolar cells, mesangial cells etc. The concentration of haemoglobin varies from person to person but usually, males have a higher level of Hb than females.
Content: Haemoglobin
Definition of Haemoglobin
Haemoglobin can be defined as a pigment-protein found in the erythrocyte cells and imparts a red colour due to the presence of iron as a central metal ion. The iron molecule is not only responsible for the colour of erythrocytes but also maintains its shape. Any abnormality in the configuration of haemoglobin can change the shape of RBCs, thereby impeding their function and flow in the circulatory system.
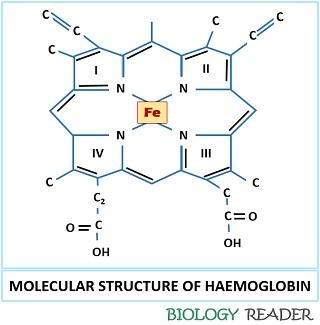 The name itself clears that the haemoglobin is composed of haem (non-protein group) and globin (protein group). The haem part of haemoglobin comprises a porphyrin ring containing Fe2+ as a central metal ion. The globin part of haemoglobin is composed of a globular protein and having four polypeptide chains.
The name itself clears that the haemoglobin is composed of haem (non-protein group) and globin (protein group). The haem part of haemoglobin comprises a porphyrin ring containing Fe2+ as a central metal ion. The globin part of haemoglobin is composed of a globular protein and having four polypeptide chains.
Structure and Functions of Haemoglobin
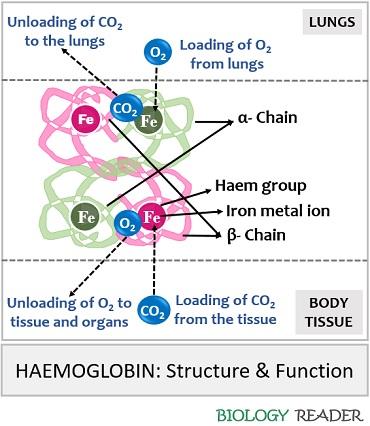
Haemoglobin is a tetramer molecule found within the RBCs consisting of four polypeptide subunits, namely two α and two β subunits. A monomer of α subunit carries 141 amino acid residues, and a monomer of β subunit carries 146 amino acid residues. Haemoglobin is a globular protein having a 3D structure which was introduced by a scientist named Max Perutz in the year 1959 through X-ray crystallography.
The physical property (like colour and shape) and the function of RBC (Loading and unloading of gaseous molecules) are due to the chemical group found in it that refers as “Haemoglobin”. Haem is a non-protein region of Hb that contains iron embedded towards the centre of the heterocyclic ring or porphyrin ring. In the porphyrin ring, four pyrrole rings combine via methyl bridges.
Iron in a porphyrin ring exists either in Fe2+ and Fe3+ state. The iron molecule attaches covalently to the polypeptide chain by coordinating with all the four N-atoms. In Fe2+state only, the binding of oxygen can be achieved. When oxygen binds with the central metal ion, the position of Fe2+ changes approximately 0.4Å.
Existence of Hb
The structure of haemoglobin exits in two states:
T-state: It is the tense state of haemoglobin that lacks the oxygen molecule and exits as deoxygenated form. During this state, Fe2+ lies outside the plane of heme. It is also called “Deoxygenated haemoglobin”.
R-state: It is the relaxed state of haemoglobin that contains an oxygen molecule and fully exits as oxygenated form. During this state, Fe2+ moves into the heme’s plane. It is also called “Oxygenated haemoglobin”.
Functions
A scientist named Claude Bernard first explained the functional role of haemoglobin, and it performs the following tasks:
- The haemoglobin protein contains an active central metal ion (Fe2+) that gives a red colour to the erythrocytes.
- It also performs gaseous exchange by loading O2 from the lungs to the rest of the body and loading CO2 from the body tissues back into the lungs.
- Hb also helps in the transportation of nitric oxide, where it binds to the thiol groups within the globin chains and forms a complex “Nitrosothiol“. This complex later dissociates again into nitric oxide and thiol at the time of oxygen release from the haem group.
Measurement Methods
The concentration of haemoglobin can be known after performing the methods given below:
- Haematocrit method
- Sahli’s method
- Drabkin’s method
Haematocrit Method
In this method, collect the blood sample in a tube with EDTA or some other reagent that prevent blood from coagulating. Thoroughly mix the blood with an anticoagulant and then dip the haematocrit tube. Allow the blood to rise to 2/3rd or 3/4th in a haematocrit tube. Then take out the haematocrit tube and wipe out the outer surface by using blotting paper. Invert the haematocrit tube and allow the blood to flow towards the tube’s bottom.
Seal the end of the haematocrit tube and place it in a microhaematocrit centrifuge for about 3-5 minutes. By the help of a haematocrit reader, measure the length of the RBCs column and divide it by the column of blood containing WBCs, platelets and plasma. After dividing, we will get the ratio of RBCs and blood containing cells and plasma that is multiplied by 100. The concentration of haematocrit in men is usually 40-54 % and 36-48% in women.
Haematocrit level = RBCs/Blood (Blood cells and plasma) X 100
Sahli’s Method
In this method, take N/10 Hydrochloric acid solution into the haemoglobin pipette up to the yellow mark (20). Then take the blood from veins up to the volume 20 µl of haemoglobin pipette. Allow the pipette to stand for 10 minutes so that the hydrochloric acid will convert the haemoglobin content of the blood into acid “Haematin”. The haematin appears dark brown and diluted until it matches the standard comparator block. Once, the dilution is completed, the concentration of haemoglobin can be determined by taking the readings of the calibration tube.
Drabkin’s Method
It is also called the “Cyanomethaemoglobin method”. It is a colourimetric method that measures the concentration of haemoglobin based on the absorption of light. In Drabkin’s technique, collect the blood sample in a tube added with an anticoagulant. Then, mix the blood sample in a Drabkin’s reagent containing a solution mixture of potassium ferrocyanide and potassium cyanide. Later, it results in a lysis of RBCs.
As a result of haemolysis, the haemoglobin content of RBCs will release into the solution. First, the potassium ferrocyanide in the solution will react with haemoglobin and convert it into “Methaemoglobin”. Then, potassium cyanide reacts with the compound Methaemoglobin and gives hemiglobinocyanide or cyanomethaemoglobin.
At last, the solution is checked for the absorption of the coloured complex at a wavelength of 540nm. A standard graph is prepared between the concentration of haemoglobin versus absorption of light in a horizontal (X-axis) and vertical (Y-axis). A straight line will obtain, and the values obtained are compared with the haemoglobin standard (Chemkit).
Important Facts About Haemoglobin
Globin chain of Hb: In infants, two α and two γ globin chains are present in the structure of haemoglobin, but when they reach the adolescence stage, the γ-subunit is replaced by β-subunit.
Iron central ion: The four molecules of Fe2+ binds with four molecules of oxygen and form “Oxyhaemoglobin” which is loaded from the lungs and transferred to the rest of the body parts. Similarly, the active Fe2+ binds to the carbon dioxide formed inside the body and unloads it to the alveoli of the lungs from where it expells out of the body.
Dietary supplement: Our diet should be supplemented with pulses, green leafy vegetables, fruits that are rich in iron, as it helps the body to make haemoglobin. Regular tea intake before and after the meal can cause iron deficiency in the body.
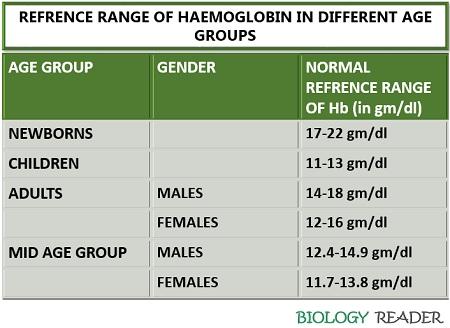
Hb concentration: The concentration of Hb varies from person to person depending upon age, gender etc.
Oxygen affinity: The binding affinity of O2 with Fe2+ metal ion depends upon the pH (low pH will decrease the O2 affinity of haemoglobin). Haemoglobin has low O2 affinity than myoglobin (A structural analogy) but acts as an efficient O2 carrier (releases O2 easily). Hb has oxygen-affinity of 1.34 oxygen per gram.
Hb colour: The colour of RBCs appear crimson red in the absence of oxygen, but on oxygenation, it appears rustic red.
Anaemia: It is a medical condition, which can occur due to poor absorption of iron, excessive blood loss, the altered shape of RBCs, vitamin deficiency etc. In some cases, the stem cells in the bone marrow, which produce RBCs are attacked by the immune system, and results in fewer RBCs leading to a medical condition refers as “Aplastic anaemia”.
Effects
Change in the haemoglobin level can cause severe issues like:
Low haemoglobin level
A person with low haemoglobin count may lead to a clinical condition known as “Anaemia” which can be caused by the following:
- Blood loss (due to any surgery)
- Minerals and the nutritional deficiency (due to lack of iron and vitamin B-12)
- Kidney failure
- Abnormal haemoglobin structure
An anaemic person suffers the following symptoms:
- Weakness
- Shortness of breath
- Dizziness
- Headache
- Pale yellow skin
- Chest pain etc.
High haemoglobin level
A person with high haemoglobin count may lead to a clinical condition known as “Polycythaemia” which can be caused by the following:
- Advanced lung cancer
- Development of tumour
- By the administration of a drug (Epogen)
- People living on high altitudes
A polycythaemic person can suffer the following symptoms:
- Thickening of blood
- Shortness of breath
- Dizziness
- heart attack and strokes
Risk factors of Anaemia: Aged people who lack iron in their diet, people doing an excessive workout, pregnant women, people living with chronic illness and females who go through excessive bleeding during the mensuration cycle.
Risk factors of Polycythaemia: People dealing with long-term lung infections are at higher risk of developing polycythaemia.