Definition: Immobilization can define as the enticement of an enzyme to a solid support. Enzyme immobilization is a process, which encloses the enzyme molecules to an absolute phase from a bulk phase. The bulk phase consists of substrates, effectors and inhibitors. Enzyme molecule impounds in or on some suitable matrix that is having a definite porosity.
The carrier or matrix allows the exchange of medium (contains substrate and product), to which an enzyme molecule can be confined. Immobilization restricts the movement of enzymes. It is a prevalent method, which is now used in many fields like industrial, medical, bioresearch, food science etc.
Therefore, the biocatalyst that gets confined to the inert material will be termed as an immobilized enzyme. The methods which facilitate the enzyme entrapment into or on the support matrix is called immobilization of an enzyme. Amino acylase was the first immobilized enzyme.
Here, we will look into some important topics related to the context like the discoveries behind the introduction of the immobilization technique, the components, physical and some chemical methods of immobilization. Besides, we will also discuss some of the advantages, disadvantages and applications of the immobilization technique.
Content: Immobilization of Enzyme
- History
- Components
- Methods
- Advantages of Immobilization
- Disadvantages of Immobilization
- Applications of Immobilized Enzymes
History
History of enzyme immobilization includes three stages in the development of an immobilized enzyme.
- First stage: During 1815, immobilized enzymes were used empirically in industrial processes, such as the production of acetic acid and wastewater treatment.
- Second stage: During the 1960s, a single enzyme immobilization system was introduced. Then this system used in the production of L-amino acids, isomerization of glucose etc.
- Third stage: During 1970-1995, multiple enzyme immobilization methods, including co-factor regeneration and cell immobilization, were introduced.
Components of Enzyme Immobilization
The major components for an enzyme immobilization include an enzyme, a support matrix and mode of attachment of a catalyst to the carrier.
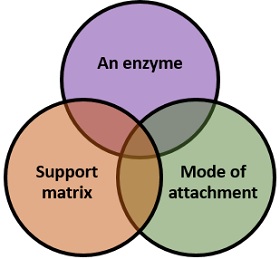
Enzyme
It can define as a biomolecule, which accelerates many biochemical reactions. It acts as a biocatalyst, which mediates the conversion of a substrate into a product, but never used up by the substrate.
Support Matrix
It is a material, which facilitates the imprisonment of an enzyme. A support matrix must have the following properties that we have discussed below for the efficient immobilization.
Ideal Properties of Support Matrix
- Mechanical strength.
- Biocompatible
- Stable
- Inertness
- Regenerability
- Ease of derivatization (transformation of the substrate into a product)
- Expansion of enzyme specificity
- Reduction in product inhibition
- Reduction of microbial contamination
- Cost-effective
Classification of a Matrix
Based on the chemical composition, a support matric is generally of two types (Organic and Inorganic carriers).
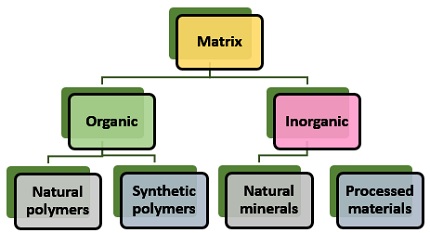
Organic matrix: It subdivides into natural and synthetic polymers.
- Natural polymers: It shows favourable compatibility with proteins. Polysaccharides (Cellulose, dextran, agar, agarose, chitin, alginate etc.), proteins (Collagen, albumin) and carbon are the natural polymers.
- Synthetic polymers: It shows high chemical and mechanical stability. Polystyrene, polyacrylate, polyacrylamide, polyamides etc. are examples of synthetic polymers.
Inorganic matrix: It subdivides into Natural and Processed minerals.
- Natural minerals: E.g.Bentonite, celite, centolite, silica, charcoal etc.
- Processed materials: E.g.Porous glass, metals and metal oxides.
Physical Characteristics of the Matrices
- Mean particle size
- Hydrophilic character
- Mechanical strength
Mean particle size: It is the parameter, which decides the porosity of a carrier. Porous matrix prefers over the non-porous matrix because it increases the surface area, by which the loading capacity of an enzyme also increases. This property of the matrix determines the total surface area and also affect the binding ability of a catalyst. A porous material should have optimized pore distribution to optimize the flow of particles.
Hydrophilic character: It determines the level of activity by an immobilized enzyme.
Mechanical strength: It can define as the binding of an enzyme that is inversely related to the ease with which it gets entrapped.
Mode of attachment
The different modes of attachment between the enzyme and support matrix are explained below in the methods of enzyme immobilization. It generally involves physical and chemical methods for the immobilization of an enzyme.
Methods of Enzyme Immobilization
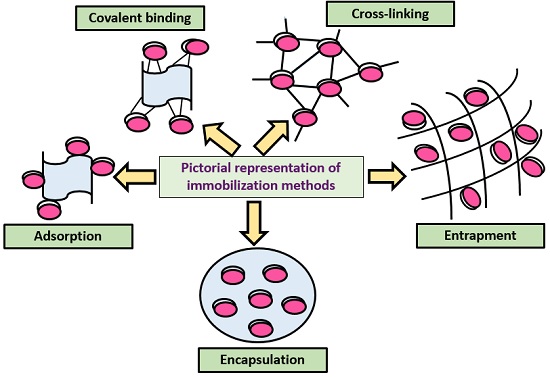
Based on the binding property, it classifies into physical and chemical processes.
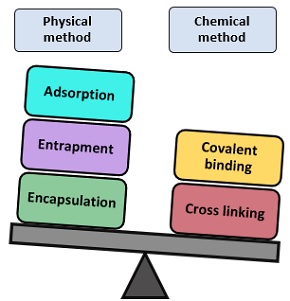
Adsorption
It is the oldest and simplest method. In this type, enzyme adheres to the surface of the water-insoluble carrier matrix. The binding is nonspecific like electrostatic or hydrophobic affinity binding to a particular ligand. The binding between enzymes and the carrier matrix is usually firm, but it gets weakened by many factors like:
- Addition of substrate
- pH or ionic strength
In enzyme adsorption, the bonding is non-permanent and accomplished by the weak bonds, mainly like hydrogen bond and Vander Waal forces.
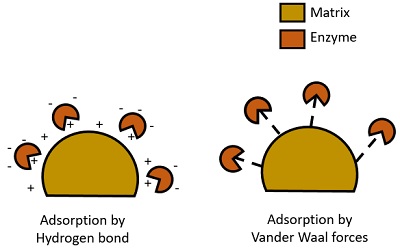
- The matrix used: The matrix’s particle size must be small (500Å-1mm D).
- Examples: Different carrier materials are used in this type like:
- Mineral support (E.g. Aluminium oxide, clay)
- Organic support (E.g. Starch)
- Modified sepharose and ion exchange resins
Methods of Immobilization by Adsorption:
- Static method: It is an efficient but time-consuming method. It involves immobilization of enzyme and carrier molecule without agitation.
- Dynamic process: It involves the mixing of an enzyme with the carrier, under constant agitation.
- Reactor loading: It involves transferring of both enzyme and carrier in the reactor with the agitation of the whole content. It is widely used for the commercial production of the immobilized enzyme.
- Electro-deposition: Here, a carrier is kept proximal to the electrode in an enzyme bath, after which an electric current is passed through it. It results in the movement of an enzyme towards the carrier. At last, the enzyme gets deposited on the surface.
Advantages of Adsorption:
- It has no pore diffusion limitation.
- It is a simple and economical method to conduct.
- No reagents are required in this method.
- There is a limited loss of enzyme activity.
- It causes less disruption to an enzyme.
- It requires minimum activation steps.
- The adsorped enzyme can be recycled, regenerated and reused.
- It has a high loading efficiency of an enzyme.
Disadvantages of Adsorption:
- It provides low surface area for the enzyme binding.
- Desorption of an enzyme from the carrier usually occurs.
- Also, the yield is low.
Entrapment
An enzyme traps inside a porous polymer or gel matrix during this method. It is also called lattice entrapment. The bonding between an enzyme and matrix can be covalent or non-covalent.
- The matrix used: It is water-soluble, and nature varies with different enzymes.
- Examples: It makes the use of the following carrier materials like polyacrylamide gels, cellulose triacetate, agar, gelatine, alginate etc.
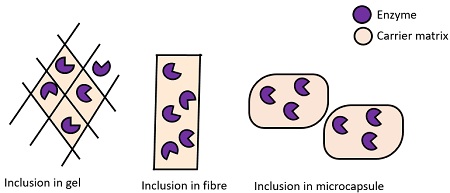
Methods of enzyme entrapment: It involves the inclusion of an enzyme into the following matrices:
- Gels: Involves entrapment of an enzyme inside the gel matrix.
- Fibres: Entrapment of an enzyme inside the fibre matrix.
- Microcapsules: Involves entrapment inside a microcapsule.
Advantages of Entrapment Method:
- Its enzyme loading capacity is high.
- It’s a rapid method.
- Here, the enzyme distortion is low.
- It is easy to practice.
Disadvantages of Entrapment Method:
- The diffusion of substrate and product create difficulties.
- It causes leakage of low molecular weight enzymes.
- There might some chances of microbial contamination.
- It also causes enzyme inactivation and sometimes loss of enzyme activity.
- It has limited industrial use.
Encapsulation
It is the membrane confinement method. An enzyme confines within the semipermeable membrane of a capsule in an aqueous solution. This process allows the exchange of medium (substrate & product), but not an enzyme. The effectiveness of encapsulation relies upon the enzyme stability.
- The matrix used: The capsule is made of a semi-permeable membrane, and it can be polymeric, lipoid, non-ionic etc. in nature.
- Examples: It includes nitrocellulose, nylon semi-permeable matrix etc.
Methods of encapsulation: It can be achieved by the following ways:
- Encapsulation in a reaction vessel: It involves the partitioning of a chamber by a semipermeable membrane. One chamber contains enzymes, whereas the other contains substrate and product.
- Encapsulation by hollow fibre membrane: It involves entrapment of an enzyme inside a semipermeable matrix (cellulose, triacetate etc.). Here, an enzyme traps inside the space of the matrix.
- Microencapsulation: By chemical polymerization, the enzyme molecules enclose inside a microcapsule by the use of 1-6-diaminohexane.
- Encapsulation by liposomes: Here, an enzyme binds to the concentric lipoidal membrane of the liposome by the use of phospholipid.
Advantages of Encapsulation:
- There is no enzyme leakage.
- It does not affect enzyme activity.
- It’s a simple method to conduct.
- It possesses a high loading efficiency of an enzyme.
Disadvantages of Encapsulation:
- It makes the use of a carrier that has a pore size limitation.
- It is not so cost-effective.
Covalent Binding
It is a widely used method. An enzyme molecule binds to the carrier by a covalent bond during this process. Here, the binding strength is powerful or a complex form through this bonding is stable. Also, there is no enzyme loss during the process. Covalent binding occurs between the active part, i.e. the functional group of an enzyme and the carrier molecule.
The functional group that are participating in the binding process are -NH2, -NH3, -COO, -OH, -SH, -O, -S etc. The order of reactivity of these functional groups to the carrier depends upon their charged status:
-S– > -SH > -O– > -NH2 > -COO– > -OH >> -NH3+
Examples of a polymeric carrier used in covalent binding are as follows:
- Carboxylic acid and related groups of polyglutamic acid
- Amide group of a polypeptide
- Amino and related groups of polysaccharides
- Some most commonly used polymers are the polysaccharide (celluloses, agarose, sepharose, etc.), polyvinyl alcohol, silica and porous glasses.
Methods used for Covalent Binding: It involves the following methods that are given below.
- Diazotation: It involves bonding between the amino group of the matrix and tyrosyl or histidyl group of an enzyme on the reaction with NaNO2 and HCl.
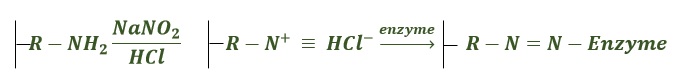
- By peptide bond: It involves bonding between amino or carboxyl group of the matrix to the carboxyl or amino group of an enzyme. During this, a matrix is chemically treated to bind with the active functional group.
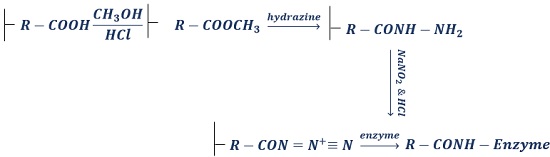
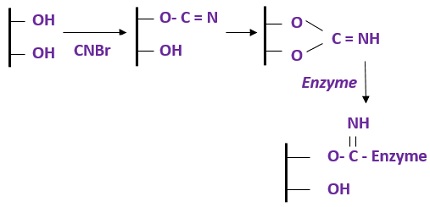
- Cyanogen bromide activation: It involves the binding of glycol groups of a matrix with an enzyme by the activation of CNBr.
- By polyfunctional reagents: This process involves bonding between the amino group of the matrix and amino group of an enzyme. Example: Glutaraldehyde (Bi-functional reagent).
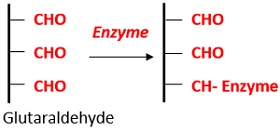
Advantages of Covalent Binding:
- It provides a high binding strength between an enzyme and a carrier.
- There is no enzyme leakage.
- It’s a simple and widely used method.
- The process is not affected by the pH or ionic strength.
Disadvantages of Covalent Binding:
- Denaturation of the enzyme occurs during the immobilization.
- Only a small amount of enzyme can be immobilized.
- It is not so cost-effective.
Cross Linking
It is also called “Copolymerization“. Here, the immobilized enzymes covalently link to the various groups of an enzyme via polyfunctional reagents. It does not require a support matrix. Cross-linking leads to the formation of 3D crosslinked aggregates. The most commonly used polyfunctional agents are glutaraldehyde and diazonium salts etc.
Advantages of Cross Linking Method:
- There is little or no enzyme leakage.
- It yields a highly stabilized enzyme.
- It’s a simple and cheap method to carry out.
- It has wide applicability in the commercial production of the enzyme.
Disadvantages of Cross Linking Method:
- It causes enzyme inactivation.
- The polyfunctional reagents used in this process generally cause enzyme denaturation.
- It is not so cost-effective.
Advantages of Immobilization
- The enzyme can be reused.
- The immobilization method minimizes the labour input.
- It increases the enzyme and substrate ratio.
- It requires a minimum activation time.
Disadvantages of Immobilization
- Its industrial use is limited.
- The enzymes may lose their catalytic property and stability.
- The enzymes get inactivated by the heat generation.
- It is expensive to conduct this method.
Applications of Immobilized Enzymes
- Industrial use: E.g. Production of antibiotics, amino acids etc.
- Biomedical use: E.g. For the treatment, diagnosis and drug delivery.
- In the food industry: E.g. Production of jams, jellies, syrups etc.
- Sewage treatment: E.g. In the treatment of sewage and industrial effluent for wastewater management.
- In the detergent industry: E.g. Immobilization of lipases to digest lipid present in stains or dirt.
- Textile industry: E.g. Scouring, bio-polishing etc.
Thus immobilization process is widely used in various fields, in which an enzyme can be immobilized to the particular phase after which it can be reused and stabilized to carry out many reactions.