The emulsion is a word that originated from the Latin word “Mulgeo”, which means “To milk”. Milk is the best example of emulsion, consisting of biphasic liquid systems containing both fat and water. The emulsion can define as the colloidal system, which includes two or more phases of immiscible liquids.
In the emulsification process, one liquid system facilitates the dispersion of the other liquid phase, and thus it also refers to as “Liquid in liquid dispersion”. It includes examples like egg yolk, milk, butter, mayonnaise etc. In other words, we can say that the emulsion is a mixture of two liquid components, which won’t mix.
It can be of two types, namely oil in water (O/W) and water in oil (W/O). The formation of emulsion strongly depends upon the volume ratio of the two liquid mixtures. Preparation of emulsion is a popular method to produce healthcare, cosmetics, food products etc.
Content: Emulsion
Definition of Emulsion
The emulsion can be defined as a two-phase system that only contains the particles in the liquid phase of matter, where both are immiscible with each other. One will disperse from the two liquid phases, and the other will be in the continuous phase. In other words, from the two phases, one liquid phase will show the dispersion of the other. Emulsions are the standard type of colloidal systems which contains two or more insoluble liquid phases. Sometimes, the term “Colloid” is interchangeable with the term emulsion.

Types
These are generally of two types, based on the continuous and dispersive phase of matter.
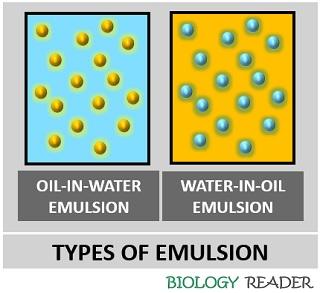
- Oil in water refers to the type of emulsion when the oil disperses in the continuous phase of water.
- Water in oil refers to the type of emulsion when the water disperses in the continuous phase of oil.
We can understand the types more efficiently by knowing the solubility of the two liquid systems. In the O/W system, oil is hydrophobic (Water hating) substance, whereas water is hydrophilic (Water-loving).
And, as per the rule of “Like dissolve like”, the hydrophobic or non-polar material will dissolve with the non-polar solvents only, not with the water. Similarly, the hydrophilic or polar material will dissolve only with the polar molecules, not lipid molecules.
Therefore, solubilizing the hydrophobic substance with the hydrophilic material is a tough task. But, by the addition of an emulsifier, the formation of an emulsion can be achieved.
Properties
Colour: Due to the Tyndall effect, emulsions usually appears cloudy or white, ON the scattering of light by the suspended particles present in the liquid mixtures.
Texture: Oil in the water system looks watery, whereas water in the oil system looks oily.
Solubility: An emulsion can be solubilized by adding liquid, which can be soluble with the continuous liquid phase.
Example: A milk can solubilize by the addition of water.
Conductivity: The oil/water system shows high conductivity, and the water/oil system shows low conductivity.
Phase inversion: It can occur by the two factors:
- By keeping emulsion for a more extended period.
- By the change in the volume ratio of the two liquid mixtures.
An emulsion is a highly unstable system, which when kept for a more extended period, can cause separation of the two phases and results in “Phase inversion”. These form homologous structures and does not have a static internal structure. It forms by agitating or emulsifying the two non-miscible phases.
Emulsifier
The emulsifier can be defined as a chemical substance that mixes the two non-miscible liquid phases and stabilizes the emulsion formation. It also refers as “Emulgent”. It maintains the stability of an emulsion by increasing the kinetic stability between the two liquid mixtures.
Examples: Surfactants, lecithin, mustard etc., are some common examples of emulsifiers.
Functions:
- Promotes the formation of an emulsion
- Reduces the interfacial tension between the two liquid mixtures
- Forms a barrier or interface between the two phases
- Aids stability to the dispersed liquid phase
Therefore, the process of stabilizing the two insoluble liquid phases to form an emulsion by either a mechanical force or by adding an emulsifier is the phenomenon commonly refers to as “Emulsification”. There are mainly two factors that promote the process of emulsification, which includes:
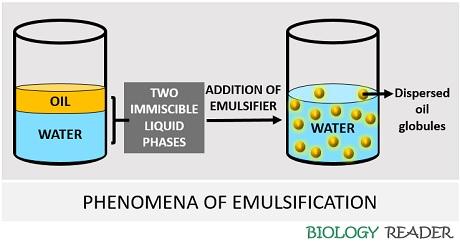
- Interfacial tension
- Viscosity
The interfacial surface tension should be less between the two liquid phases for the development of emulsion. The viscosity of both phases should be in an equilibrium state.
Applications
Milk, butter, mayonnaise etc., are prepared on an enormous scale by emulsification technique. Cod liver oil, cortisol are used in medical formulations. Cream, lotion, soap etc., are commonly prepared in the cosmetic industries.