Ozone depletion is the process of destruction of the ozone layer in the stratospheric region. It can adversely affect various living forms. Ozone depletes by the many pollutants that are emitting out from the vehicles, industries etc. Ozone layer depletion is one of the major environmental issues, which needs to be controlled as the layer is getting thinner day by day due to increased pollution.
Stratospheric ozone safeguards the living forms by absorbing the most damaging UV-B type rays that can cause skin allergies and eye damage. Oppositely, the ozone in the troposphere is considered bad, as it constitutes the primary source of greenhouse gases that can cause respiratory illness.
Before understanding the whole phenomena of ozone depletion, we will cover all the important terms and facts of ozone, the ozone layer and an ozone hole. Last but not least, we will talk over the causes, effects on the living systems and management of the ozone depletion.
Content: Ozone Depletion
- Definition of Ozone
- Generation of Ozone
- Ozone Layer
- UV Radiation
- Ozone Hole
- Causes of Ozone Depletion
- Effects
- Control
Definition of Ozone
Here, we will briefly discuss the general, physical and chemical properties that will give us the whole idea about ozone:
| Characteristics | General properties of ozone |
|---|---|
| Discoverer | Charles Fabrey and Henry Buisson in 1913 |
| Location | Stratosphere of the atmosphere |
| Distance | 16-40km from the earth surface |
| Characteristics | Physical properties of ozone |
|---|---|
| Physical state | Gaseous form |
| Colour | Blue coloured gas |
| Odour | Pungent smell |
| Characteristics | Chemical properties of ozone |
|---|---|
| Chemical formula | O3 |
| Structure | Bent |
| Polarity | It is a polar molecule |
| Solubility | More soluble in non-polar molecules like O2, CO2, CFCS etc. and less soluble in polar molecule like water |
| Dipole moment | 0.53D |
| Bond angle | 1.272Å |
| Magnetic property | Diamagnetic |
A high concentration of ozone is found in the stratosphere region, and some concentration is found in the troposphere of the atmosphere.
- O3 in the stratospheric zone safeguards us from the lethal UV rays (mostly UVB-type) and also considered as Good stratosphere ozone.
- Oppositely, O3 found in the troposphere region contributes to the atmospheric pollutants that can cause many effects like respiratory hazards (asthma, bronchitis etc.), lungs damage, headaches, burning eyes etc. and also considered as Bad troposphere or low-level ozone.
Ozone Generation
An O3 is continuously generated and dissociated in the stratosphere, and the phenomena is known as “Chapman cycle”.
Formation of ozone: It involves the following steps:
- Firstly, dissociation of the oxygen molecule takes place under the presence of UV-light of wavelength (80-240nm).
- After that, the dissociated O-atom combines with the oxygen molecule to produce ozone in the stratosphere.
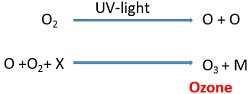
Dissociation of ozone: It involves the following steps:
Here, ozone absorbs the UV-C photon of wavelength (200-300nm) and dissociates.
![]()
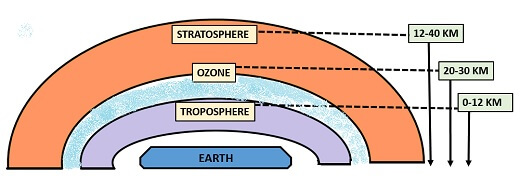
Ozone Layer
It is also called “Ozonosphere”. The ozone layer is a thin layer that acts as a protecting shield by absorbing harmful UV- radiations. It is situated in the lower portion of the stratosphere region. The ozone layer is present about 20-30km above the earth surface. It absorbs up to 97-99% of UV-light less than the wavelength of 290 nm and has a concentration of about 300-350 D.O.
The level of ozone is measured by the Dobson unit that is named after the scientist G.M.B. Dobson. He first devised the instrument, which he named “Dobson meter” (a simple spectrophotometer) to know the amount of ozone that overheads from the ground. The thickness of the ozone varies, but generally;
- Near equator: It is thinner
- Near pole: It is thicker
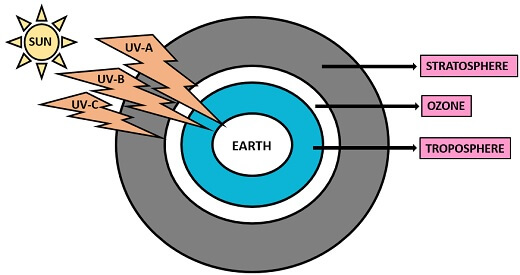
UV Radiation
It is the non-ionizing radiation and a part of electromagnetic rays, whose wavelength ranges from 100-400nm. UV radiation produces naturally by the sun in the form of solar energy. It can also be produced artificially by the mercury and tanning lamp.
It was first discovered by Johann Wilhelm Ritter in the year 1801. The UV radiation is present between the X-rays and Visible light. UV-light has wavelength more than X-ray but less than the visible light.
Classification: UV light categorizes into three types, depending upon its penetration property:
- UV-A
- UV-B
- UV-C
| Types of UV-radiation | Wavelength (in nm) | Ozone absorption | Effects |
|---|---|---|---|
| UV-A | 315-400 | 50% | Cause skin aging, wrinkles, DNA-damage leads to skin cancer. |
| UV-B | 280-315 | 70-90% | Cause skin tanning, eye cataract etc. |
| UV-C | 100-280 | 100% | This is blocked by the ozone therefore it does not show any effect. |
Ozone Hole
It was first seen in Antarctica continent in the year 1987. During that year, the ozone hole was observed between 12-24 km altitudes. Later, it was investigated that the occurrence of ozone hole occurs annually during mid of august and september in the Antarctic continent. The major factors involved in the cause of ozone hole was not known but it was predicted that chlorine and its radicals deplete ozone.
Mario Molina and Sherwood Rowland first concluded that the stratosphere has a limited power to absorb chlorine molecules. On further study, it was confirmed that the CFCs in aerosols and refrigerants were the primary cause of ozone depletion, in which one chlorine atom may disrupt thousands of ozone molecules.
Causes of Ozone Depletion
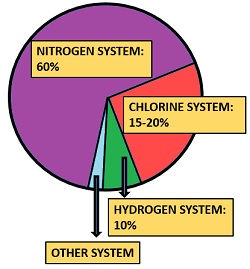
The stratospheric ozone depletes by the number of gaseous pollutants like nitric oxide (NO), nitrous oxide (N2O), chlorine (Cl), chlorofluorocarbons (CFCs) etc. These gaseous pollutants react with ozone to produce oxygen, by which the net result is the depletion of ozone. The main causes of ozone depletion are as follows:
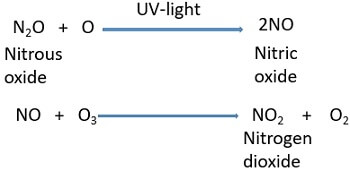
Nitrogen system: It causes 60% of ozone destruction.
Example: Nitrogen system can be explained by taking an example of nitrous oxide (N2O). Soil and ocean form nitrous oxide that first enters the atmosphere by the microbial activities and then to the stratosphere.
- In the stratosphere, NO first reacts with the nascent oxygen in the presence of UV- light to form nitric oxide (NO).
- Then, nitric oxide reacts with the ozone to produce oxygen and acts as a powerful destroyer of ozone.
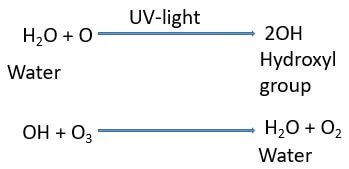
Hydrogen system: It causes 10% of ozone destruction.
Example: It can be explained by taking an example of hydroxyl (OH) group that is derived from the water molecule.
- Firstly, the water molecule reacts with the nascent oxygen to form a hydroxyl group.
- Later, a hydroxyl group reacts with the ozone and produce oxygen and water.
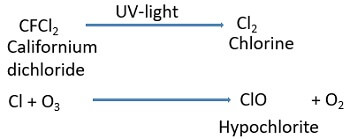
Chlorine systems: It causes about 15-20% of ozone destruction.
Example: It can be explained by taking the example of CFCs.
- Firstly, CFCl2 in the presence of UV light dissociates to form chlorine (Cl2).
- Then, a Cl2 reacts with the ozone to produce oxygen.
Other systems: Halons are some other compounds that can also deplete ozone. It primarily includes chemical compounds that are widely used in the fire extinguishers.
Examples: Bromoflourocarbons, Bromochloroflouro carbons etc.
Effects of Ozone Depletion
It is a matter of concern that can cause detrimental effects to the living organisms. Let us take a quick view into the effects of ozone depletion on humans, plants and aquatic systems.
Effects on human health: Thinning of ozone can cause skin and eye damage in humans like:
- Skin cancer (sometimes refers as “malignant melanoma”)
- Eye cataract by direct exposure
- Skin ageing
- The weakening of the immune system
- Skin infections
- tanning of skin
Effects on plants: The depletion of ozone affects the growth and development of the plant such as:
- Reduced growth of a plant
- Reduction in the efficiency of photosynthesis
- Production of smaller leaves
- Premature death
- Discolouration of leaves
Effects on the aquatic ecosystem: Thinning of the ozone layer may drastically affect the balance of the aquatic ecosystem by the following factors like:
- Decrease the photosynthetic efficiency of phytoplankton
- Effect development stages of fish, shrimp and other aquatic forms.
- Unbalance the marine ecosystem
- Indirectly interferes with the marine food chain
Control of Ozone Depletion
The management of ozone depletion includes the following measures:
- Spread awareness about the ozone depletion.
- There should be restricted use of CFCs.
- Atomic nuclear explosions should be banned as they emit nitric oxide.
- The eco-friendly household cleansing product must be used.
- Rocket flight and high altitude aircraft should be minimized.
- Unleaded gasoline should be used in the vehicles.
- Vehicles should be equipped with a catalytic converter.
- CFCs should be replaced with HCFC.
Depletion of ozone is a serious environmental issue and it drastically increases by the increased concentration of the environmental pollutants like nitrogen and chlorine radicals. As a result, the ground-level gaseous pollutants deplete the ozone concentration in the stratosphere. Some major steps should be taken to minimize or control the thinning of this protective layer.
Its gonna be really harmful for everyone . People must be aware of this. Making people aware regarding this issue is a great job done by you. I am glad for your work.