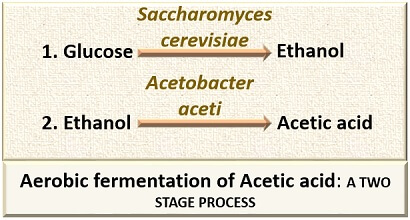
Acetic acid production refers to the production of acetic acid or vinegar through two steps modification via two different microorganisms. The first step involves the conversion of sugar into ethanol by the yeast (mainly Saccharomyces cerevisiae). In the second step, oxidation of ethanol into acetic acid takes place by the bacteria like Acetobacter, Gluconabacter sp. and the process termed as “Acetification”.
In the ancient time, vinegar was naturally produced from the wine opened to the air. This cause spoilage of wine or “Souring of wine”, and the vinegar produced by this natural method is called “sour wine”. In the 19th Century, modern methods like surface and submerged fermentation were introduced for the large scale production of acetic acid.
Technically, a vinegar must contain about 4% of acetic acid per 100ml to call it as “Legal vinegar”. In this context, you will get to know the definition, production process (through a synthetic and biological method), recovery, biosynthesis and uses of the acetic acid.
Content: Acetic Acid Production
- Definition
- Production Process of Acetic Acid
- Recovery of Acetic acid
- Biosynthesis
- Uses of Acetic acid
Definition
Acetic acid production employs a synthetic and biological method to produce acetic acid. The synthetic process involves some chemical reactions to produce acetic acid, whereas a biological method involves microorganisms that degrade the substrate into a product (acetic acid).
A biological process initially requires Saccharomyces cerevisiae for the conversion of the sugar-containing substrate (like molasses) into the ethanol and finally makes the use of Acetobacter aceti, which brings about the transformation of ethanol into acetic acid.
What is Acetic Acid?
Acetic acid or “Ethanoic acid” is a weak organic acid. Some of the important properties of acetic acid are mentioned below in the table:
| Properties | Acetic acid/ Vinegar/ Ethanoic acid |
|---|---|
| Solubility | Miscible in polar solvent like water |
| Chemical formula | CH3 - COOH |
| Pure form | In pure form i.e. without water refers as “Glacial acetic acid” |
| Functional group | One carboxylic acid present as functional group and also refers as “Monocarboxylic acid” |
| Taste and Smell | Sour taste with a pungent smell |
| Boiling point | 391 K |
| Freezing point | 290 K |
Production Process of Acetic Acid
Acetic acid can be produced by the following two methods like:
- Synthetic method
- Biological method
Synthetic Method
A synthetic method for acetic acid production involves catalytic oxidation of acetaldehyde. To obtain acetic acid by the synthetic method, acetylene goes through catalytic hydration to form acetaldehyde, and later acetaldehyde undergoes catalytic oxidation to give acetic acid.
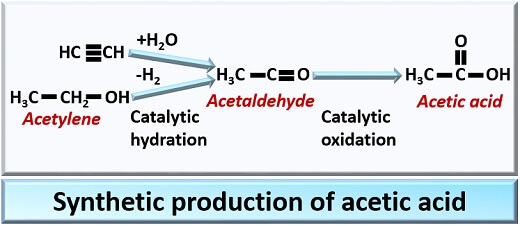
The acetic acid formed by the synthetic method is called “Synthetic vinegar”. The synthetic vinegar is then subjected to the processes like dilution, aromatization and colouration.
- Dilution: The synthetic vinegar is first diluted via the addition of water.
- Aromatization: The aromatization of synthetic vinegar involves the addition of sugar, salts and a small amount of natural vinegar.
- Colouration: At last, the vinegar is coloured via the addition of caramel.
A synthetic vinegar must be diluted in a way, that it must contain 4% of acetic acid per 100ml of water. The concentration of acetic acid will decide the strength of vinegar. The strength of vinegar is measured in grams and termed as “Gram strength”.
Biological Method
The biological method is generally employed for the commercial production of acetic acid through microorganisms like yeasts and bacteria. There are two methods, namely surface and submerged fermentation process widely used for the large scale production.
The fermentation of acetic acid can be anaerobic or aerobic. The anaerobic fermentation is carried out using Clostridium sp. of bacteria (in the absence of oxygen). Aerobic fermentation is primarily carried out by the yeasts (Saccharomyces cerevisiae) and secondly by the bacteria (Acetobacter aceti).
In the case of substrates like malt, whey etc. of low alcohol content, there is no need to add additional nutrients. If substrates like potato, grain spirits are used, then nutrient must be added for the optimal growth of microorganisms and acetic acid production.
Surface Fermentation
It uses trickling generator for the acetic acid production.
Construction of trickling generator: The outer surface of the tank is composed of wood with the carrying capacity of 60m3. The inner surface contains the birch of wood shavings. Below the column of wood shaving, there is a cooling system at the bottom, which controls the heat generation during the process. The collecting chambers collect the liquid and recirculate it to the top for the conversion of ethanol into acetic acid. The acetic acid is drawn off through the outlet.
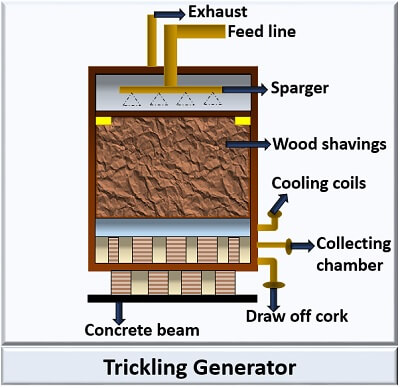
Process: First, the ethanol is transferred into the generator from the inlet pump at the top. The ethanol is trickled through the column of wood shavings. The bacteria is inoculated at the bottom, which converts the ethanol into acetic acid through their microbial activity. A solution is cooled and pumped back to the top and again passed through the birch of wood shavings. The process is repeated until 80-90% of the ethanol converts into acetic acid.
The concentration of ethanol should not be less than 0.2%, as it can increase the death rate of the microorganisms producing acetic acid. Therefore, for the optimum growth of microorganisms and production of vinegar, the concentration of ethanol must be in between 0.2-5.0%. The significant advantage of surface fermentation is that it leaves little residue with a high yield of acetic acid.
Submerged Fermentation
In the submerged fermentation, the substrates with low alcohol content like fruits and wines are used in the initial step, under anaerobic conditions. Nowadays, high yielding substrate and productive strains are used to produce 13% of acetic acid, under 50m3 oxygen.
Construction of the submerged fermentor: The submerged fermenter is made up of “Stainless steel”. The effigas turbine, at the bottom, allows continuous agitation. A suction rotor of the fermentor provides aeration. The heat exchange plates control the temperature of the culture medium. There is a foam separator, which eliminates the foam formed in the culture vessel. Through the harvester pump, the acetic acid is taken out.
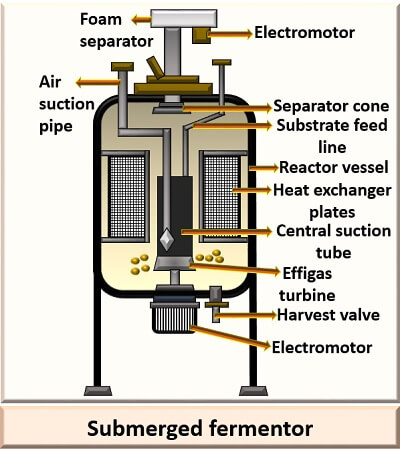
Process: Firstly, the ethanol is inoculated with the bacterial strain Acetobacter acetigenum. The fermentation is carried out for 35 hours at 40 degrees Celsius. The bacteria then grow and ferment the ethanol to acetic acid through continuous agitation and aeration.
Submerged fermentation gives 10times/m3 higher yield than the surface fermentation and it is a cost-effective method. It requires only 20% of the plant area for the installation of the machine and does not require labour skills because of the automated machines.
Recovery of Acetic acid
In the recovery stage, the fermented product is recovered to obtain pure acetic acid. The acetic acid is produced along with the water and certain by-products like formic acid, bacterial biomass, which must be separated. The separation of heavy particles like bacterial mass can be done either by the method of centrifugation or filtration.
For the filtration method, plate filters are extensively used. After the filtration, the acetic acid is subjected to decolouration by adding of K4 [Fe (CN) 6]. Distillation is also employed for the separation of water and acetic acid.
Biosynthesis
The biosynthesis of acetic acid includes three chemical modifications.
- Oxidation of Ethanol: First, the ethanol is converted into acetaldehyde catalyzed by the alcohol dehydrogenase in the presence of NAD+ molecule.
- Hydration of Acetaldehyde: The acetaldehyde undergoes hydration reaction where it converts into acetaldehyde hydrate.
- Oxidation of Acetaldehyde hydrate: In this reaction, acetaldehyde hydrate further oxidizes by the acetate dehydrogenase to produce acetic acid in the presence of NADP molecule.
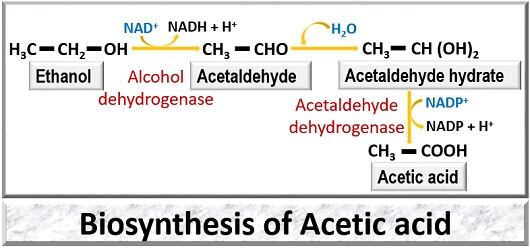
Therefore, acetic acid is a product formed as a result of partial or incomplete oxidation of ethanol.
Uses of Acetic acid
The acetic acid includes many applications like:
Rubber industry: Acetic acid acts as a coagulant or binding agent in manufacturing rubber.
Dyes preparation: Uses in the manufacturing of dyes and inks.
Fibre industry: Acetic acid helps in manufacturing the rayon fibres.
Clinical use: Acetic acid acts as an antiseptic against bacteria like Pseudomonas, Streptococci etc. and it is used in the treatment of the outer ear infections caused by bacteria and fungus.
Food industry: In the food industry, acetic acid acts as condiment or preservative that is added in the pickles, sauces, raw vegetables etc.
Perfume industry: Acetic acid is also used in the preparation of perfumes.
Very useful information.