Chlorination is a disinfection method used in the wastewater treatment plant. It helps in the destruction of microorganisms from the running wastewater. Microorganisms can be pathogenic or non-pathogenic.
Wastewater contains pathogenic enteric microorganisms that impose severe health problems. Typhoid, cholera, dysentery etc., are common waterborne illnesses. And wastewater treatment aims to reduce the possibility of this happening.
Like other treatment processes, there are several methods available to achieve disinfection. The methods include chlorination, ultra-violet (UV) light, ozonation and bromine chloride additions. Among all, chlorination is the most common.
The chlorination method uses different forms of chlorine to disinfect the treated wastewater. Chlorine has been used popularly as a primary water disinfectant. It is largely responsible for reducing the incidences of waterborne diseases.
Considering human health, the destruction of pathogens is necessary to make wastewater reusable. This post describes the meaning, process, advantages and disadvantages of chlorination. Besides, we will discuss different forms of chlorine used in chlorination.
Content: Chlorination in Wastewater Treatment
- Meaning
- How Chlorine Destroys Microorganisms?
- Factors Affecting Chlorination
- Disinfection of Treated Wastewater
- Why Chlorine in Wastewater Treatment?
- Chlorine Properties
- Chlorine Compounds as Disinfectant
- Advantages
- Disadvantages
- Conclusion
Meaning of Chlorination
Chlorination is the chemical disinfection of treated wastewater by using chlorine. This method removes pathogens and prevents waterborne illnesses.
- The pathogenic organisms include viruses, bacteria and protozoans.
- Cholera, dysentery, typhoid etc., are waterborne illnesses.
Chlorination is essential to disinfect the water or make the water supplies potable. It is the stage before the water runs off into oceans, rivers and streams.
How Chlorine Destroys Microorganisms?
Chlorine compounds destroy microbes by oxidising their cellular material. After oxidation, the cell membrane weakens, and chlorine enters the cell. As a result, it disrupts cell respiration and DNA activity. And, we know how these two processes are necessary for cell survival. So, by disrupting cell functionality, chlorine destroys different microbes.
Factors Affecting Chlorination
Effective chlorine disinfection depends on the following:
- Water’s pH levels (The effective disinfection occurs when the water is at a lower pH).
- Temperature and nature of water.
- Chlorine type and concentration.
- The nature and number of microorganisms.
- Contact time (the time the chlorine is in the water).
- Levels of ammonia and suspended solids.
- The presence of reducing agents (decrease chlorination efficiency).
Disinfection of Treated Wastewater
Disinfection of wastewater includes a variety of methods like:
- Chemical Method: It includes chlorination, ozonation, etc.
- Physical Method: It involves ultraviolet radiation, microfiltration, etc.
- Biological Method: It includes detention lagoons.
This post is about one of the chemical disinfection methods for the wastewater, which is “Chlorination”.
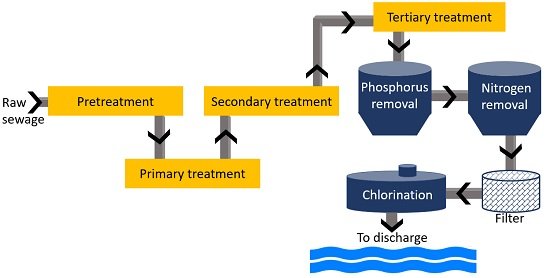
Chlorination
The STP treats the wastewater coming from agriculture, sewage and industrial plants. The treatment process has four stages (pretreatment, primary, secondary and tertiary treatment).
Before chlorination, wastewater must be free of all contaminants (inorganic and organic wastes). The primary and secondary treatment eliminates 85% of organic matter. Tertiary treatment disinfects wastewater by reducing pathogens harmful to living beings.
The treated water requires disinfection before:
- Environmental discharge
- For drinking purposes.
At advanced or tertiary treatment, chlorine is added to public drinking water. Chlorination involves adding a measured amount of chlorine to the treated wastewater.
In reaction with water, chlorine residues form. These residues are efficient to kill bacteria, viruses, and cysts. It is followed by an upstream filtration step. This stage removes sediment that could bind to chlorine.
Ozonation: It uses the formation of free radicals as oxidising agents. Ozonation is more effective against viruses and bacteria than chlorination.
The low solubility of ozone in water reduces its disinfection capacity. Ozone residual dissipates as a consequence of its reactive nature.
Why Chlorine in Wastewater Treatment?
- Chlorine is easily available as gas, liquid or powder.
- It is less expensive.
- Chlorine compounds are easy to apply due to their high solubility in water.
- The residues of chlorine are not potent to man.
- Being strong oxidising agents, they are toxic to various microorganisms.
- Chlorine performs foul air scrubbing.
- It helps in treating phenols and cyanides wastes.
- It controls activated sludge bulking.
- Also, chlorine stabilises the waste activated sludge.
- It can remove ammonia from the water.
- Application of chlorine in STP controls filter flies and foaming.
- It assists in removing grease and scum from the water.
- It mitigates odours and keeps septicity at bay.
Chlorine Properties
- Chlorine exists as a gas at room temperature and pressure.
- It is water-soluble.
- It acts as the best chemical disinfectant for the disinfection of treated wastewater.
- Hydrolysis occurs when chlorine reacts with water. And, the reaction gives hypochlorous acid and hydrochloric acid.
- Cl2 + H2O → HOCl + HCl
- Sodium hypochlorite reacts with water as follows:
- 2NaOCl + H2O → NaOH + NaCl
- Chlorine reacts with water to produce hypochlorous acid (HOCl). And, hypochlorous acid dissociates to form the hypochlorite ion.
- HOCl ↔ OCl- + H+
- Other forms of chlorine:
- Monochloramine (NH2Cl)
- Dichloramine (NHCl2)
- Chlorine as disinfectant causes:
- Oxidation of cell walls leads to cell lysis.
- Inactivation of functional sites on the cell surface.
- Chlorine level up to 4 milligrams per litre is safe for drinking purposes.
Chlorine Compounds as Disinfectant
We can add chlorine into the tertiary system via chemical feed inlets. Three standard formulations of chlorine are available for chlorination:
- Gaseous chlorine (useful in larger public water treatment plants)
- Liquid sodium hypochlorite (bleach solution)
- Solid calcium hypochlorite (Dry powder or pellet)
Chlorine Gas
It is a greenish-yellow gas. It has tremendous use as a disinfectant. Some drinking water facilities use chlorine gas. Its use has some advantages and several potential disadvantages. Gaseous chlorine is a good oxidising agent.
Advantages
- Chlorine is the most effective disinfectant to deactivate waterborne pathogens.
- It offers economical disinfection.
- Also, it occupies less storage space in comparison to chlorine solutions.
Disadvantages
- It is a toxic respiratory irritant.
- Specific reactions cause chlorine gas explosions.
- Wet chlorine is highly corrosive.
- It requires special storage and handling considerations.
Due to these disadvantages, STPs consider some other alternatives that are as follows:
Sodium Hypochlorite (NaClO)
It is also known as liquid bleach. Sodium hypochlorite is a chlorine derivative that is a light yellow liquid. It is much safer than chlorine gas.
A lower concentration (5-15%) of NaClO is effective for water disinfection. This concentration is more effective than chlorine gas or calcium hypochlorite.
The sodium hypochlorite (NaClO) and water reaction give hypochlorous acid and hypochlorite ions. When these combine, free available chlorine (FAC) forms that act as the disinfectant.
It is widely used in low-hardness water. By treating high-pH sodium hypochlorite with high-hardness water, calcium salts forms. Also, it promotes scaling.
Though it is safer than chlorine gas, some precautions should be considered:
- Depending on the chlorine concentration, it can cause skin, stomach, and throat irritation.
- It is quite unstable and affected by heat, light, pH and metal contamination. Each factor reduces effectiveness and relative shelf life.
Calcium Hypochlorite (Ca(ClO)2)
It is manufactured from chlorine gas. Calcium hypochlorite is a solid or dry form. It exists as white pellets or granules. Calcium hypochlorite contains 65% of concentrated chlorine.
It has a much higher chlorine concentration than NaClO, resulting in higher costs. Calcium hypochlorite offers lower upfront costs than the above two disinfectants.
Calcium hypochlorite is stable and effective. It can be hazardous if subjected to heat or stored near an oxidised organic material.
Advantages of Chlorination
- Chlorine becomes a choice of wastewater treatment plant because it is less expensive.
- Efficient in killing most bacteria and viruses in water.
- Gives residual protection against recontamination.
- Ease-of-use and acceptability.
- Helps in reducing diarrheal disease incidences.
Disadvantages of Chlorination
- Less effective against protozoa.
- Lower effectiveness in disinfection of turbid waters.
- The application of chlorine has a potential taste and odour objections.
- By-products of chlorination have potential long-term effects.
Conclusion
Chlorination is a common way to disinfect water and kill waterborne pathogens. Nowadays, a physical mode (UV disinfection) is common to disinfect municipal wastewater.
Free and combined chlorine residues form (toxic to aquatic organisms) during chlorination. Also, there are chances of the formation of organo-chlorinated derivatives. These derivates are of particular concern, as they are toxic, persistent and bioaccumulative.
Dechlorination is also important to remove combined chlorine residues left after chlorination. But, it does not affect chlorinated organic compounds present in the final discharge.