Refining of metal is one of the processes which involves the separation of impurities like sand particles, other metals etc. from the metal of choice. Therefore, refining of metal comes under the science of studying the metal’s physical and chemical properties, called Metallurgy.
The methods for refining metals vary according to the type and use of the metals. Refining merely refers to as purification. Thus, metal refining removes the gangue particles or matrix from the metals obtained through the various reduction processes and gives a pure or refined metal.
The process of metal refining is carried out after the extraction of metal. The metals’ refining process involves adding some substances that can confer desirable characteristics to the metals. Here, we will discuss the meaning, properties, types, occurrences of metals and its different refining processes.
Content: Refining of Metal
What are the Metals?
Metals are defined as the elements that are solid, hard, lustrous, and they have good conductance over electricity and heat. The metals have the following properties like:
- Physical appearance: Metals are hard, crystalline and opaque. Some metals are coloured, while some are not.
- Malleability: Metals are malleable, i.e. it can mould into various shapes.
- Ductility: Metals are ductile, i.e. it can transform into thin wires.
- Lustrous: Metals are shiny in appearance.
- Conductivity: Metals acts as a good conductor of heat and electricity.
Types of Metals
According to the periodic table, the metals can be categorized into:
- Alkali metals: These belong to the elements in column 1 of the periodic table. These metals comprise six elements, namely Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs) and Francium (Fr). These metals strongly react with water to produce alkali, due to which they are also called alkali metals.
- Alkaline earth metals: These belong to the elements in column 2 of the periodic table. Alkaline metals comprise six elements, namely Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). These metals react with the molecular oxygen and form alkaline compounds, or we can say compounds with basic nature.
- Transition metals: These belong to the elements from column 3 to column 12 of the periodic table. Transition metals comprise fourty elements from column 3- 12 and called d-block elements and comprise twenty elements in the lanthanide and actinide series called inner transition metals or f-block elements.
- Rare earth metals: It comprises of fifteen elements, and from the name, it is clear that these kinds of metals rarely occurs in nature. Rare earth metals contain elements from Lanthanum to Lutetium plus Scandium and Yttrium.
- Poor metals: These metals comprises of fourteen elements.
- Semimetals: They are also called metalloids. The property of semimetals is interlinked between the property of metals and non-metals. Semi-metal comprises of nine elements, namely Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), Polonium (Po), Iodine (I) and Astatine.
Occurrence of Metals
In nature, the metals can occur in both Free State and Combined state.
- Metals in a free state: Metals that occur in free- state have least chemical reactivity as gold, silver, mercury etc. and called noble metals.
- Metals in a combined state: They can combine with air, moisture, CO2, non-metals etc. and form compounds like oxides, carbonates, halides etc.
Therefore, the metals are defined as the elements present in a mineral of the ore, from which it can be further extracted and refined.
Methods for Refining of Metal
The impurities like unwanted metals, non-metals, an unreduced oxide of a metal, flux, slag etc. from the crude metals are eliminated to produce pure metals. The following methods are generally employed in the metal refining:
- Liquation
- Polling
- Distillation
- Electrolytic refining
- Zone refining
Liquation
In liquation, the easily fusible metals or the metals with a low melting point like tin, lead etc. are commonly used. The process mainly involves the following steps that can be summarized into:
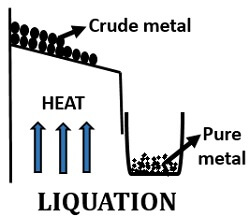
- Pouring of impure metal: In this step, pass the impure metals over the sloping hearth of the reverberatory furnace.
- Heating: Then, heat the metal at a temperature little above the melting point.
- Drain off: Drain out the refined or pure metal by leaving the infusible impurities.
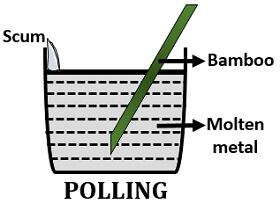
Polling
In the process of polling, stir the impure molten metal with the help of bamboo. The hydrocarbons will reduce the metal oxides present as an impurity. This method can refine metals like copper (Cu) and tin (Sn).
Distillation
By the process of distillation, volatile metals like Zinc (Zn) and Mercury (Hg) or the metals possess a very low boiling point are generally used. The metal easily vaporizes by leaving behind the impurities. In this, heat the impure metal at a temperature above its melting point in a reverberatory furnace. Then, reconvert the vapours into the metal after the separation of gangue particles.
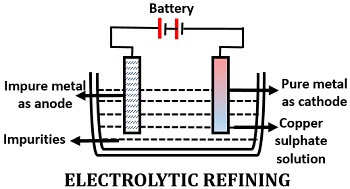
Electrolytic Refining
It is the most widely used method, as it can be used to purify many metals. It works on the principle of electrochemical properties of the metals. The impure metal is of an anode, pure metal is of a cathode, and the electrolytic solution contains the salt of the same metal used.
Under the electric field, the metals’ impurities get dissolved from the anode (gets thinner), after which the pure metals get deposited at the cathode (gets thicker). The impurities generally deposit at the anode base as sludge or anode mud.
Zone Refining
William pfann first gave this method. Some inert gases are filled in the container to which the impure metals are kept inside. Then, place a circular heater at the top of the rod.
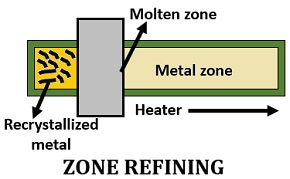
The impure metals heat up due to the circular heater. The pure metal crystallizes, and later it is cooled by the shifting of the heater to the next zone. The molten impurities will then move to the next zone along with the heater, which we can collect or separate from the last zone.