An acid-base titration is one of the titration methods. First and foremost, we should know what titration is and what it requires to conclude the results. A titration is a quantitative chemical analysis that helps in determining an unknown concentration of a solution by the known concentration of a solution.
It must be clear that acid and base are the two reactants taking part in the acid-base titration. It is a type of volumetric analysis. Using acid-base titrations, we can determine the unknown concentration of acid or base through the known concentration of acid or base.
Acid-base titration involves the neutralization of an acid and a base, also called neutralization titration. Like titration, it depends on a chemical reaction of the analyte with a standard reagent.
This post describes the meaning, principle and important terms used in the titration theory. You will get to know the definition, types, requirements and procedure of acid-base titration. Also, the titration curve for different acid-base titrations has been explained.
Content: Acid-Base Titration Theory
- What is Titration?
- Important Terms
- Acid-Base Titration Definition
- Types
- Requirements
- Ideal Properties
- Procedure
- Calculation
- Conclusion
What is Titration?
Titration is also called “Titrimetry”. It is a chemical method that helps us determine the concentration of an unknown sample by the known concentration of the standard solution. Titration is a kind of volumetric analysis that involves volume measurements.
A titration method mainly requires a burette and conical flask.
- A burette holds the standard solution whose concentration is known.
- A conical flask holds the analyte or sample whose concentration is unknown.
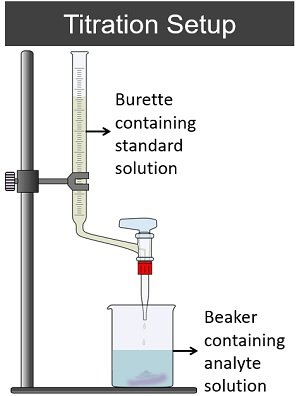
Titrant (standard solution) is a reactant added to the titrand (sample to be analyzed). Then, the characteristics of the solution change once the mole concentration of the titrant and titrand become equal.
An indicator determines a change in the solution, usually in the form of colour changes. At last, we can calculate the volume of titrant added to the titrand.
Important Terms
- Titrant or Titrator is a standard solution whose concentration and volume are known.
- Titrand is an analyte or sample whose concentration and volume are unknown. It is the solution to which a titrant reacts.
- Titration volume is the volume of titrant reacted with titrand.
- An indicator is an organic dye that determines the equivalence point by changing the colour of the solution.
- An equivalence point or stoichiometric point is when the amount of added titrant is equal to the amount of analyte being analyzed.
- The endpoint is when the colour changes in a system by indicating that the titration has been completed.
Acid-Base Titration Definition
It is a quantitative titration method. Acid-Base titration depends on the neutralization reaction between an acid and a base in a solution. In this, one of the solutions is acid, and the other is a base. It uses acid-base indicators that indicate the endpoint of titration by changing colour.
Principle: The principle of acid-base titration is relatively simple. If you know the stoichiometry of a reaction and the quantity of one species, you can calculate the quantity of the other.
The concentration of an analyte can be calculated just by recording the endpoint. An indicator is generally used to note down the endpoint or the final reading in the graduated burette.
Types of Acid-base Titrations
Acid-base titrations estimate acids or bases with unknown concentrations using a standard acid or alkali solution.
- Acidimetry estimates the concentration of an alkali solution using a standard acid solution.
- Similarly, alkalimetry determines the concentration of an acid solution using a standard alkali solution.
Based on the strength of acids and bases, acid-base titrations are of the following types:
- Strong acid-Strong base
- Weak acid-Strong base
- Strong acid-Weak base
- Weak acid-Weak base
Before explaining the types of acid-base titrations, we must know what the titration curve is. We can determine the pH of the solution at the equivalence point through a titration curve. It indicates the change in the pH of the analyte solution as the titrant is added from the burette.
The titration curve is a plot between the volume of titrant dripped into the analyte versus the pH of the analyte solution.
- X-coordinate indicates the volume of titrant added before starting the titration.
- Y-coordinate represents the pH of the analyte solution during the titration.
The solution’s pH at the equivalence point depends upon the strength of acids and bases that react in the titration.
- Strong acid-strong base titration (pH = 7 at equivalence point)
- Weak acid-strong base titration (pH > 7 at equivalence point)
- Strong acid-weak base titration (pH < 7 at equivalence point)
- Weak acid-weak base titration (pH varies)
Following are the types of acid-base titrations:
Strong Acid-Strong Base Titration
In this, the strong acid and strong base dissociate entirely to give a neutral solution. At the equivalence point, hydronium (H+) and hydroxide (OH–) ions combine to give water.
For this reason, the pH is always 7 at the equivalence point between the strong acid and strong base. This kind of titration uses a phenolphthalein indicator, as you can quickly notice the colour change.
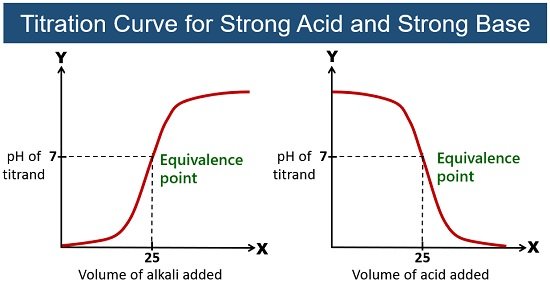
Example: Titration of hydrochloric acid (HCl) with NaOH. The reaction between the two gives neutral salt (NaCl) and water.
Weak Acid-Strong Base Titration
Here, the reaction of a weak acid with a strong base reacts in a one-to-one ratio. At the equivalence point of a weak acid and strong base titration, the pH is larger than 7. Phenolphthalein is more proffered for this titration.
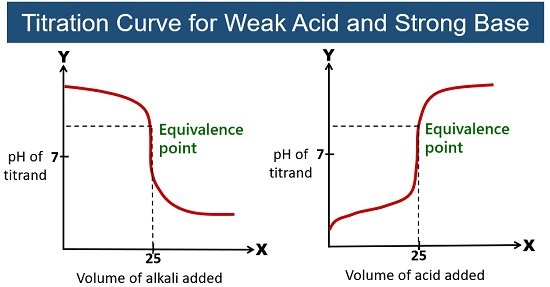
Example: Titration of acetic acid (CH3COOH) with NaOH. This reaction gives sodium acetate (CH3COONa) and water.
Strong Acid-Weak Base Titration
Here, the acid and base react to give an acidic solution. A conjugate acid formed during the titration gives a proton or hydronium ion to the base. At the equivalence point of a strong acid-weak base titration, the pH is less than 7. Methyl orange is preferred for this titration.
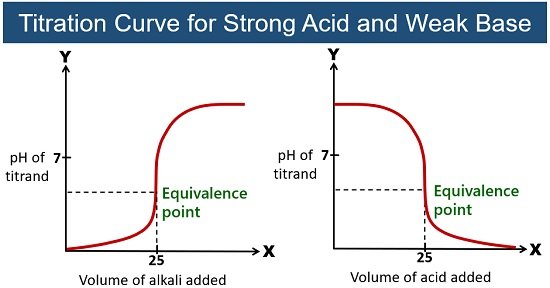
Example: Titration of hydrochloric acid (HCl) with ammonia (NH3). This reaction forms a neutral salt, ammonium chloride (NH4Cl).
Weak Acid-Weak Base Titration
Here, the acid and base react to give a slightly basic solution. A conjugate base is formed during the titration. As the OH– reacts with the H+ in the acetic acid during this titration, the acetate ion (C2H3O2–) is formed.
This conjugate base reacts with water to form a slightly basic solution. In this titration, the pH gradually changes around the equivalence point (with a pH greater or less than 7).
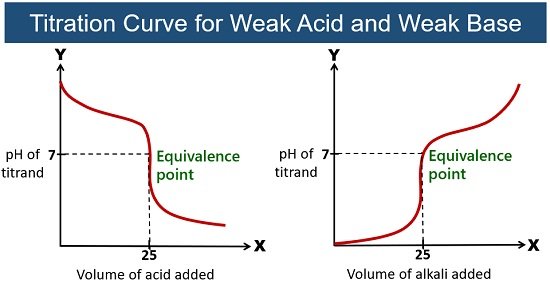
Example: Titration of acetic acid (CH3COOH) with ammonia (NH3). This reaction forms a neutral salt, ammonium acetate (CH3CO2NH4).
Requirements
- A long-graduated burette with a stopcock
- Burette stand
- Conical flask or Beaker
- Funnel
- Indicator-dye
Ideal Properties
- A titrant should thoroughly react with the analyte.
- A titrant should rapidly react with the analyte.
- The completeness of the reaction must be greater than 99.9%.
- To prepare standard solutions in the acid-base titration, always uses strong acids (HCl, HClO4, H2SO4 etc.) or strong bases (NaOH, KOH etc.). Weak acids and bases do not react completely with analytes, for which they are not used to prepare the standard solution.
Video: Acid Base Titration
Procedure of Acid-Base Titration
Steps involved in the acid-base titration involve:
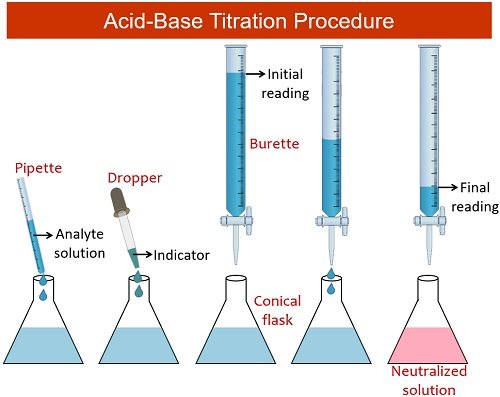
- First, you need to clean and dry the conical flask, burette and funnel required to perform the experiment.
- Then, fill the burette with a standard solution or titrant of known concentration using a funnel.
- After that, accurately measure the volume of the analyte solution or titrand and add it to the conical flask.
- Afterwards, add several drops of indicator using a dropper to the flask containing the sample of unknown concentration.
- Then, carefully titrate the sample in the flask by allowing single drops to fall into the flask.
- Titrate the sample with the titrant or standard solution until the indicator changes the colour of the solution.
- When the indicator permanently changes the colour, note down the endpoint.
- Repeat the titration for three more trials, record the initial and final readings in the observation table and calculate the volume of titrant added into the flask.
Calculation
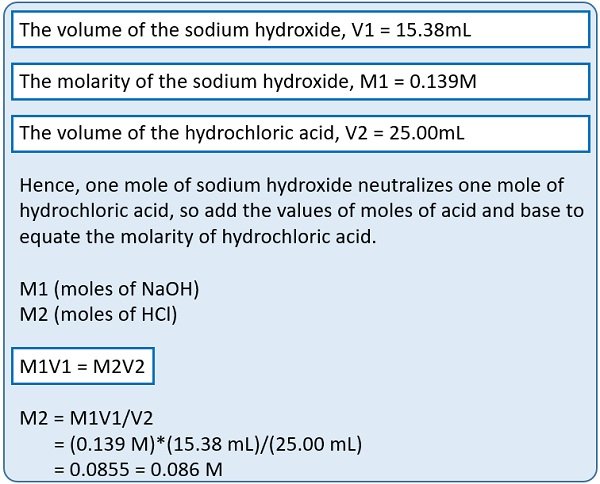
Let us take an example to calculate the concentration of an unknown sample by knowing the values of other variables.
Example: In a titration experiment, 15.38 mL of 0.139M NaOH neutralizes a 25.00 mL sample of HCl. What is the molarity of the HCl sample?
Conclusion
Therefore, titrations are commonly used for measuring unknown concentrations. Using titration, we can predict the unknown concentration of an analyte through a solution of known concentration.
Its primary purpose is to find out the equivalence point of the solution, where the two, i.e. an acid or a base, react in a predictable manner. The endpoint of the titration is when the colour of the solution changes, and it gives the volume of the titrant dripped into the flask.
Much clear information.