The difference between colloid and solution is due to the properties like the solubility of particles, chemical nature and light scattering property. Colloids form when the particles (diameter from 1-1000 nm) are suspended in the dispersion medium. Oppositely, a true solution forms when the solute particles (diameter less than 1 nm) completely dissolve in the solvent.
Dispersed particles in the dispersion medium are responsible for the heterogenecity of the colloidal system because more than one phase appears. Conversely, the solubility of solute particles in the solvent makes the solution a homogeneous mixture, or only a single-phase appears.
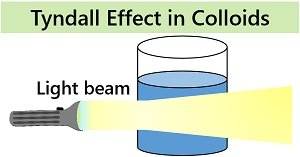
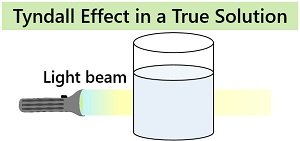
Light scattering or the Tyndall effect is an optical property that mainly distinguishes colloids and solutions. Only colloids exhibit a light scattering property due to uniformly dispersed particles. Solutions do not show the Tyndall effect, or a light beam completely passes through the homogeneous mixture.
The solubility of solutes in the solvent has a varying affinity. Thus, depending upon the degree of affinity between particles, colloids and solutions are the resulting mixtures. This post describes the key differences between colloid and solution, along with the comparison chart. You will also learn the definition, properties, types, examples and similarities between the two.
Content: Colloid Vs Solution
Comparison Chart
| Properties | Colloid | Solution |
|---|---|---|
| Meaning | It is a mixture containing dispersed or suspended particles in the dispersion medium | It is a mixture containing one or more solutes in the solvent |
| Components | Dispersed phase and dispersion medium | Solute and solvent |
| Phase | It consists of more than a single phase | It consists of a single phase |
| Nature | Heterogeneous mixture | Homogeneous mixture |
| Appearance | Cloudy | Clear |
| Particle size | It usually ranges from 1 nm to 1 mm | It is less than 1 nm |
| Visibility of particles | Particles are invisible to the naked eye but visible under the electron microscope | Particles are invisible to the naked eye |
| Light penetration | Translucent | Transparent |
| Diffusion | Particles slowly diffuse | Particles rapidly diffuse |
| Sedimentation | Particles may settle down through centrifugation | Particles do not settle |
| Interaction between the particles | Colloid particles interact through electrostatic interaction, excluded volume repulsion, steric forces and Vander Waals forces | Solute and solvent interact through attractive intermolecular forces like hydrogen bonding, ion-induced dipole forces and Vander Waals forces |
| Tyndall effect | It shows Tyndall effect | It does not exhibit Tyndall effect |
| Brownian motion | Colloid particles exhibit Brownian movement | No such movement exists |
| Filtration | Particles are separable through the parchment filter | Particles are not separable through ordinary and parchment filters |
| Examples | Milk, blood, ice-cream etc. | Air, honey, soda water, brine solution etc. |
Definition of a Colloid
A colloid is a heterogeneous mixture with suspended solute particles in the gas, liquid or solid dispersion medium. Thus, a colloidal system has two distinct phases. A dispersed phase contains suspended particles, and a continuous phase is the suspension or dispersion medium.
The suspended particles in the dispersion medium have a diameter from 1 nm to 1 mm. Colloids are also known as “Colloidal dispersions” due to dispersed colloid particles in the continuous phase. Let us look into some common examples of colloids.
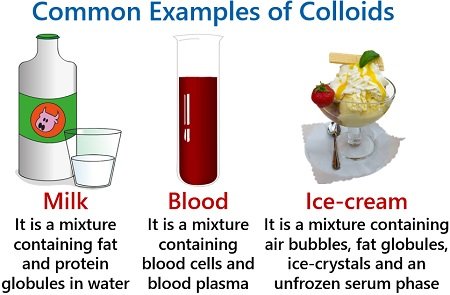
Milk is the colloidal mixture containing dispersed liquid fat and protein globules within the water. Blood is also an example of a colloid, which contains blood cells (RBCs, WBCs and platelets) and blood plasma. Then, ice cream is a colloidal mixture containing fat globules, air bubbles and ice crystals.
Definition of a Solution
A solution is a homogeneous mixture with one or more solute particles in the solvent phase (either gas, liquid or solid). Thus, a solution is a mixture containing two components (solute and solvent). The solute is a compound that dissolves in a solvent, whereas the solvent functions as the dissolving medium.
The solute particles in the dissolving medium or solvent have a diameter of less than 1 nm. A solution is also known as a “True solution” due to its homogeneous nature. Let us look into some common examples of solutions.
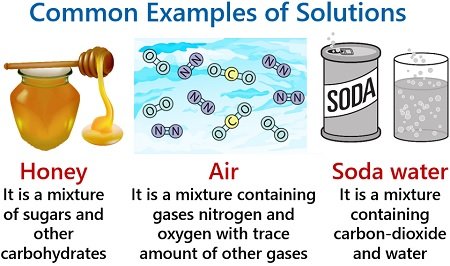
Honey is a homogeneous mixture containing sugars (fructose and glucose) and other carbohydrates. Air is a mixture comprising gases like nitrogen and oxygen with other gases in trace amounts. Soda water is a solution containing carbon dioxide and water.
Properties of a Colloid
Physical property: A colloid is a heterogeneous mixture with two or more phases. The particle size of colloids is intermediate between the solution and suspension (generally ranges between 1 nm-1 mm).
Suspended particles are immiscible in the dispersion medium. Colloids have a unique feature by exhibiting Brownian movement (dispersed phase particles move in a zig-zag pattern due to constant collisions.
Optical property: Colloids possess a light scattering property or exhibit the Tyndall effect. Uniformly suspended particles of colloidal solution reflect or scatter the light coming into its path. Through this property, we can differentiate between a true solution and a colloidal solution.
Chemical property: Repulsive forces stabilize the colloidal dispersion and prevent particles coagulation. Colloid particles interact via excluded volume repulsion, electrostatic interactions, steric forces and Vander Waals forces.
Mechanical property: Colloid particles are pretty stable or do not settle down at the bottom upon standing. However, through centrifugation, the colloidal particles sediment at the bottom. Dispersed particles of a colloidal system penetrate the filter paper but not the parchment paper.
Properties of a Solution
Physical property: A solution is a homogeneous mixture containing solute and solvent. The particle size of solutes in a solvent is generally less than 1 nm.

Solute particles are miscible in the solvent or dissolving medium. A true solution possesses solute and solvent phases, in which a solvent phase decides the physical state of a solution (solid, liquid or gas).
Optical property: A true solution has no Tyndall or light-scattering effect. A beam of light is invisible and unreflected when passed through the homogenous mixture.
Chemical property: Solute and solvent molecules interact through attractive intermolecular forces like hydrogen bonding, ion-induced dipole forces and Vander Waals forces.
Mechanical property: A true solution is stable, or solute particles do not settle down at the bottom upon standing. Solute particles of a true solution quickly diffuse through the filter paper and membrane filter like parchment paper.
Types and Examples
The classification and examples of colloids are given in the table based on the dispersed and continuous phase particles.
- Sol contains solid particles in a liquid or solid dispersion medium.
- The emulsion contains dispersed liquid particles in the liquid or solid dispersion medium.
- The foam contains gaseous particles within a liquid or solid phase.
- Aerosol comprises a dispersed liquid or solid particles in the gas phase.
| Type of Colloid | Dispersed Phase | Dispersion Medium | Example |
|---|---|---|---|
| Sol | Solid | Liquid | Paint, Ink |
| Solid sol | Solid | Solid | Ruby glass |
| Emulsion | Liquid | Liquid | Milk, oil in water |
| Solid emulsion/gel | Liquid | Solid | Cheese |
| Foam | Gas | Liquid | Whipped cream |
| Solid foam | Gas | Solid | Lava, pumice |
| Aerosol | Solid | Gas | Smoke |
| Aerosol | Liquid | Gas | Fog, mist |
The classification and examples of solutions are given in the table based on the solvent phase particles.
- Solid solutions is a mixture containing solid, liquid or gaseous solute particles in the solid solvent phase.
- Liquid solutions is a mixture containing liquid, solid or gaseous solute particles in the liquid solvent phase.
- Gaseous solutions is a mixture containing gaseous solute particles in the gaseous solvent phase.
| Solute | Solvent | Solution | Example |
|---|---|---|---|
| Solid | Solid | Solid | Alloys (brass) |
| Liquid | Solid | Solid | Mercury dental amalgam |
| Solid | Solid | Solid | Air bubbles in ice cubes |
| Liquid | Liquid | Liquid | Alcohol in water |
| Solid | Liquid | Liquid | Sugar in water |
| Gas | Liquid | Liquid | Soda water |
| Gas | Gas | Gas | Air |
Key Differences Between Colloid and Solution
- A colloid is a mixture containing dispersed or suspended particles in the dispersion medium. In contrast, a solution is a mixture containing one or more solutes in the solvent.
- Dispersed phase and dispersion medium are the two components of colloids, whereas a solution is a mixture containing solute and solvent.
- Colloids consist of more than a single phase and are considered a heterogeneous mixture. Oppositely, solutions only consist of a single-phase and are considered a homogeneous mixture.
- The particle size of colloids ranges from 1 nm to 1 mm, due to which particles are only visible under the electron microscope and filterable through the parchment paper. The solute particles of a solution are less than 1 nm, due to which particles are invisible to the naked eye and unfilterable through both ordinary filter paper and parchment filters.
- Colloids diffuse and sediment at a lesser speed than the particles in a solution and suspension, respectively.
- Colloids exhibit Brownian motion and Tyndall effect, whereas a true solution does not show such properties.
Similarities
- Particles in the solution and colloidal system are not visible to the naked eye.
- Separation of colloid particles and solute particles is not possible through the ordinary filter media.
- Colloid particles and solute particles do not sediment by the effect of gravity.
Conclusion
Solutions and colloids are the resulting mixtures, depending upon the variable solubility of suspended and solute particles in the dissolving medium and dispersion medium.