Water is a solvent that dissolves a variety of substances. It is considered the universal solvent because it can dissolve compounds (like polar molecules and ions) than the available solvents. Its physical and chemical attributes make it an excellent solvent.
The chemical formula of water is H2O, i.e. it contains two H-atoms and one O-atom. The atoms are held together via a polar covalent bond due to the uneven distribution of electrons.
It gives a partial negative charge to an O-atom and a partial positive charge to the H-atoms. Water can dissolve any substance with charged particles or electronegative atoms.
Compounds can be hydrophilic or hydrophobic based on their solubility in water. “Hydrophilic compounds” like polar molecules and ions can dissolve in water. In contrast, “Hydrophobic compounds” like fats and oils do not dissolve in water.
This post describes the physical and chemical characteristics of water that make it an excellent solvent. Also, you will get to know the examples to understand each property of water better.
Content: Water as a Solvent
Chemical Properties of Water
The polar nature, dissociation and ionizing properties of water are ideal for a good solvent.
Polar Solvent
Two hydrogen atoms and one oxygen atom of H2O share electrons by forming a covalent bond. But, one pair of electrons is shared unevenly between two atoms, or the sharing is not equal.
Oxygen being more electronegative attracts electrons more strongly in comparison to H-atoms. Thus, oxygen is an electron-loving atom compared with hydrogen.
A total of four pairs of electrons are around the oxygen atom, in which:
- Two pairs of electrons take part in covalent bonding with H-atoms.
- The oxygen atom carries two lone pairs of electrons when it bonds with the hydrogen atom. The other two unshared pairs lie on the opposite side of the O-atom.
The unequal sharing of electrons gives a partial negative charge to an O-atom and a partial positive charge near H-atoms. For this reason, water is considered a polar molecule.
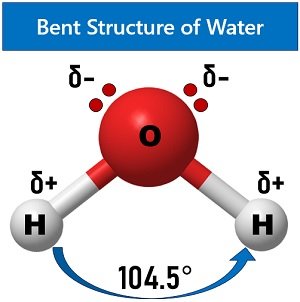
The shared or unshared electrons repel each other, bending the O-H bond away from the linear angle. For this reason, water has a bent structure.
Dissociation
Above, we have discussed the polarity of water. Now it’s time to study the property of dissociation or hydrolysis of compounds by water. Water molecules in an aqueous phase will interact or form a weak intermolecular bond.
The formation of a weak hydrogen bond between water molecules is due to a partially positive hydrogen atom and a more electronegative oxygen atom.
As the opposite attracts, the positive H-atom will make a bond with the negative O-atom. Similarly, the negative oxygen atom will bond with the positive hydrogen atom.
Thus, the attraction of the positive towards the negative allows water molecules to interact, making a cohesive structure. The water’s partial charges cause the dissociation of various polar molecules or ions, making them soluble in water.
- Water dissociates ionic salts into their respective cations and anions.
- Water dissolves many polar biomolecules because they are hydrophilic.
Dissociation of complex compounds gives off simpler constituents (atoms or ions). So, water as a solvent can solvate other polar and ionic compounds.
The charges associated with these molecules form hydrogen bonds with water. Then water molecules surround the dissociated ions by forming a sphere of hydration.
The hydration shell is a structure, in which water molecules encircle ions in an aqueous solution. This shell keeps ions dispersed in the water.
Dissociation of Ionic Compounds
Ionic compounds or salts like NaCl, NaOH etc., are polar in nature. Unlike water molecules, ionic compounds have an ionic bond. Once polar ionic compounds react with water, they dissolve or give off their ions to become a part of a solution.
Due to stronger covalent O-H bonds in water, the ionic bonds in salt molecules break, by giving ions in water. Then individual ions interact with the polar regions of water by forming a hydration sphere.
Examples
When NaCl reacts with water, it dissociates into its respective ions. The slightly electropositive ion attracts toward the electronegative O-atom of water.
At the same time, the slightly electronegative ion attracts the electropositive H-atom of water. The attraction between the dissociated ions and water molecules set up a “tug-of-war“.
The stronger covalent O-H bonds in water win out, causing ionic compounds to pull apart. Finally, anions and cations of ionic compounds are set loosely in place between the intact water molecules.
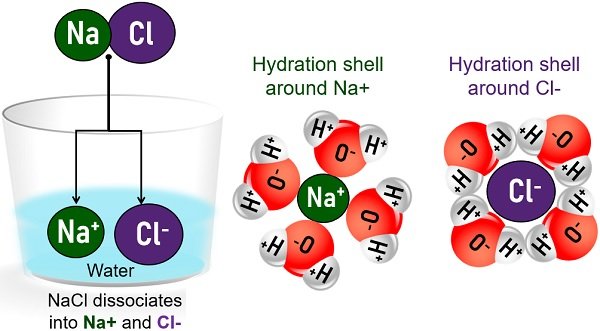
NaCl crystals react with water and dissociate into Na+ and Cl– ions. Later, a sphere of hydration forms around the ions.
- The partially negative O-atom of water forms a sphere around the positively-charged sodium ion.
- The partially positive H-atoms form a hydration shell around the negatively-charged chloride.
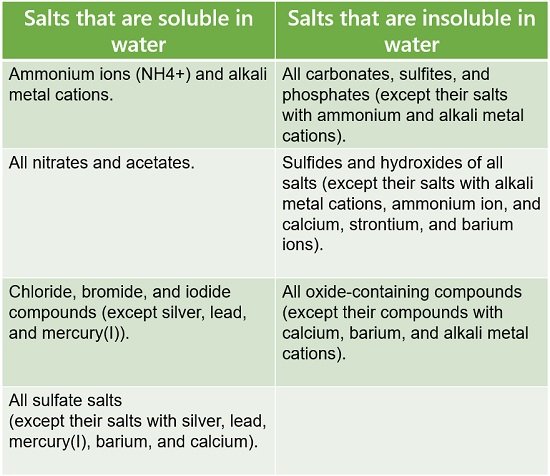
This interaction dissolves NaCl in water or causes solvation of NaCl. We can predict the solubility of salt by following a set of empirical rules:
Dissociation of Polar molecules
Many polar molecules are either polar or charged. They act as hydrophilic molecules and are easily dissolved in water. But, compounds like oil, waxes and lipids contain hydrocarbon chains that repel water molecules.
Hydrophilic Interactions
Polar molecules (like HCl, NH3 etc.) have covalent bonds with unequal charge distribution. Their atoms have differing electronegativities and are thus soluble in water.
They have dipoles on their atoms that interact with the dipoles on water molecules. Polar covalent molecules include ammonia, hydrochloric acid, ethanol, methanol, glucose etc.
Examples: Ammonia is a polar covalent molecule that is very much soluble in water. It has one nitrogen atom covalently bonded to three hydrogen atoms.
High electronegativity towards the nitrogen atom creates a polar covalent bond. The nitrogen atom has a negative dipole, and each hydrogen atom has a positive dipole.
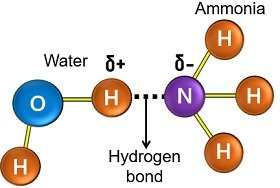
It allows the interaction of a nitrogen atom with water via a hydrogen bond. The ammonia dissolves in water and forms a basic solution.
A small amount of dissolved ammonia reacts with water to form ammonium hydroxide. Later, the ammonium hydroxide dissociates into ammonium and hydroxide ions.
Ethanol is also a polar compound that can be soluble in water. Its -OH group is hydrophilic (water-loving), while the C2H5 group is lipophilic (fat-loving). The partial positive H-atom of water interacts with the -OH end by forming a hydrogen bond.
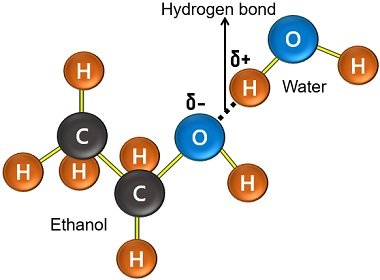
Hydrophobic Interaction
It occurs between the water and hydrophobes (nonpolar or low water-soluble molecules). Hydrophobes have a long chain of carbons that are hydrophobic (water-hating).
Example: Interaction of fat or oil and water.
Fat molecules clump together and have minimal contact with water. When nonpolar molecules react with water, the water changes its hydrogen bonding patterns around the hydrophobic molecules. Eventually, this interaction forms a cage-like structure called a clathrate.
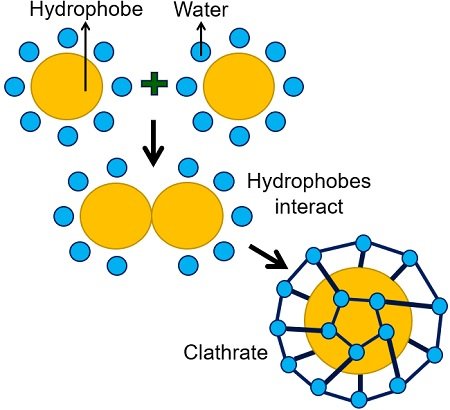
This causes the system’s overall entropy to decrease significantly, which is not spontaneous. For this reason, the hydrophobic molecule will not dissolve. Thus, water is a poor solvent for hydrophobic molecules (lipids).
Ionizing Solvent
Pure water exhibits a property of self-ionization. Water molecules can easily break up into H3O+ and OH– ions in the following way:
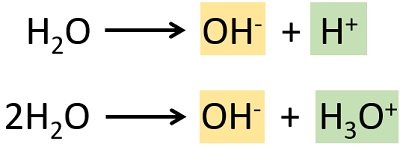
Here, the H3O+ and OH– ions are produced in equal amounts with the same concentration. The property of self-ionization is also known as auto-pyrolysis of water.
The above reaction represents the amphoteric nature of the water, in which water can act as an acid (donates proton) and a base (accepts proton).
- The H-atom of each H2O molecule carries a slight positive charge.
- The O-atom of H2O molecules has a slight negative charge.
The property of self-ionization in water helps in the dissociation of ionic compounds into cations and anions.
Physical Properties of Water
Properties like high dielectric constant, high association property, low viscosity and neutral nature of water make it a good solvent.
High Dielectric Constant
Water has a high dielectric constant (78.4) at room temperature than the other solvents. Due to the high dielectric constant, the attraction force between water’s opposite ions is maximum. This property of water helps it to polarise easily.
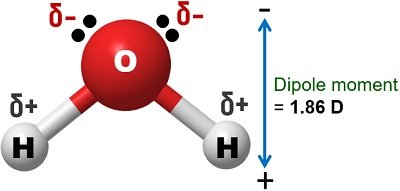
Water has a maximum dipole moment (1.84 D) than other solvents. It has a permanent dipole moment due to the presence of O-H bonds. Water can cause the ionization of polar compounds and ions.
Association Property
Water has a high affinity of association, as they happily interact with each other through intermolecular H-bonding. This increases the effective surface area of the water molecule and results in a high boiling point.
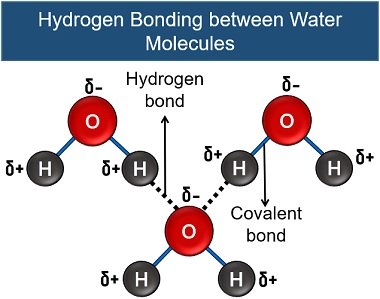
So, the water can exist as a liquid state in a wide range of temperatures (0 to 100 degrees Celsius). Thus, this property also makes the water a suitable solvent.
Viscosity
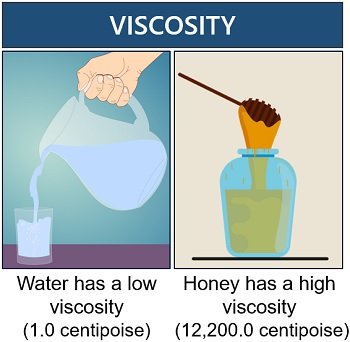
Viscosity is a measure of a fluid’s resistance to flow. Low viscous solvents are considered best than high viscous solvents. Particles coagulate easily in solvents with high viscosity, and they can not be resolved or reused. Water has a low viscosity of 1.0 centipoise at a temperature of 20 degrees Celsius.
Neutral Solvent
Water is an electrically neutral molecule because of the same concentration of H+ and OH– ions. A water molecule has a zero net charge due to 10 protons and 10 electrons. It has a positive area at one end and a negative area at the other, thus contributing to the polar nature.
Thus, the polarity of water makes it suitable to form charge-based interactions with other polar molecules and ions. Polar molecules and ions interact with the partially positive and partially negative ends of water.
Conclusion
Besides the property we discussed above, water is readily available in nature and is non-poisonous. These two ideal properties also make water a good solvent.