Acid-fast staining was originally pioneered by a scientist named Paul Ehrlich in the year 1882. Later, it was modified by Ziehl and Neelson in 1883. Thus, acid-fast staining is also called Ziehl Neelson staining. It is a type of differential staining method used to distinguish between acid-fast and non-acid-fast bacteria.
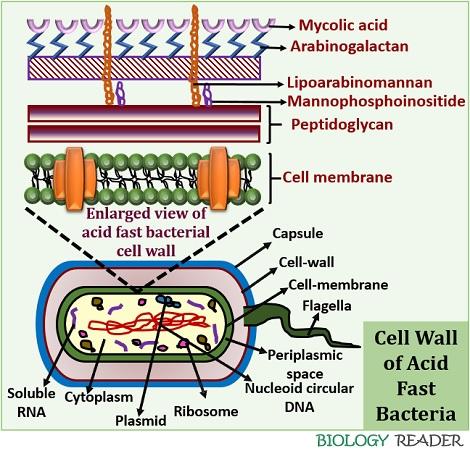
Mycobacterium is an acid-fast bacterium which retains the colour of carbol fuschin even after treatment with a decolourizer. The mycobacteria species retain the primary stain’s colour because they contain mycolic acid in their cell wall.
Mycolic acid is a waxy substance that does not allow the decolourizer to enter the cell wall due to its waxy nature. Therefore, acid-fast staining discriminates the mycobacterium species from the other groups of bacteria.
Non-acid fast bacteria quickly lose the primary stain’s colour due to the absence of mycolic acid and appear blue. This post discusses the definition, requirements, principle, process, observation and result of acid-fast staining.
Content: Acid Fast Staining
Definition of Acid-Fast Staining
Acid-fast staining refers to one of the staining methods, which differentiates the Mycobacteria species from the other bacterial groups based on the staining properties and cell wall differences. The cell wall of Mycobacterium species contains mycolic acid, which makes it resistant towards the effect of acid decolourizer and thereby called the “Acid-fast bacteria”. In contrast to this, non-acid fast bacteria lack mycolic acid in their cell wall and lose the primary stain’s colour.
Red-coloured acid-fast bacteria indicate the positive result of acid-fast staining. Oppositely, blue-coloured non-acid fast bacteria indicate the negative result of acid-fast staining.
- Examples of acid-fast bacteria: Mycobacterium tuberculosis, M. leprae, M. smegmatis, M. phlei etc.
- Examples of non-acid fast bacteria: Escherichia coli, Staphylococcus aureus etc.
Requirements of Acid-Fast Stain
Acid-fast staining is a differential staining procedure which uses a combination of three reagents:
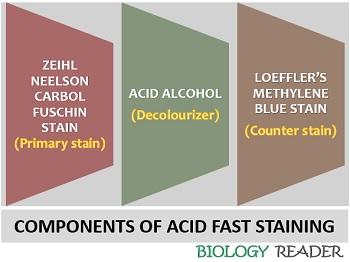
- Ziehl Neelson Carbol fuschin
- Acid alcohol
- Loeffler’s Methylene blue
Ziehl Neelson Carbol Fuschin (ZNCF)
It functions as a Primary stain. To stain a mycobacterium, one needs a special stain like ZNCF. An ordinary stain cannot stain the mycobacterial cells. ZNCF stain has a phenolic base and a carbol fuschin dye. A phenolic base of ZNCF shows a high affinity toward the lipid content.
Thus, ZNCF dye can solubilize the lipoidal material in the cell wall and stain the cell red. Due to high lipid content, a mycobacterial cell wall is less permeable. So, a phenol base increases the cell permeability, by which a cell allows the stain to penetrate.
Acid Alcohol
It serves as a decolourizing agent, which contains 3% of HCL along with 95% ethanol. It plays an essential role in the identification of acid-fast and non-acid-fast organisms. Acid-fast bacteria contain a high lipid content, which prevents the cell from binding with stains and decolourizers.
Thus, acid-fast bacteria will retain the colour of the primary stain and appear red. In contrast to this, non-acid fast bacteria lack a large amount of lipid content. As a result, the cells lose the colour of primary stain and decolourize.
Methylene Blue
It functions as a counterstain and contains 3% of methylene blue. Methylene blue stains the decolourized cells of non-acid fast bacteria and makes them appear blue. Unlike non-acid-fast, acid-fast bacteria will not take up the colour of methylene blue and appear red.
Principle
Acid-fast staining is based upon the principle of staining the bacterial cell relative to their cell wall differences. The cells of Mycobacteria species appear red, whereas non-acid fast bacteria appear blue. A smear is subjected to heat after staining with Zeihl Neelson Carbol fuschin. During steam, carbol fuschin penetrates the bacterial cell. All the cells appear red after 2-3 minutes because a primary stain (carbol fuschin) is added at several intervals during the steaming process to prevent cell drying.
After this, a decolourizer (3% HCl) is added to the smear. Now, this is an important step that helps us to distinguish between the mycobacteria and the other bacteria. The mycobacteria resist the effect of decolourizer because of the substantial lipoidal material or mycolic acid in the cell wall. Thus, mycobacteria are considered acid-fast bacteria, as they don’t allow the penetration of the acid decolourizer into the cell, and remains appear red.
On the contrary, other bacteria will not resist the effect of decolourizer due to little or no lipoidal content. Here, the other bacteria are considered non-acid fast bacteria, as the decolourizer causes leakage of primary stain by creating pores in the cell wall. Thus, a non-acid fast cell appears colourless.
Only the decolourized or non-acid fast cells will take the blue colour of methylene blue stain on counterstaining. Conversely, the acid-fast cells will remain red in colour.
Video: Acid Fast Staining
Procedure
To carry out the process of acid-fast staining, we require the following:
Requirements: Clean glass slide, slide holder, bacterial suspension, inoculating loop, Bunsen burner, distilled water, water bath, ZNCF, acid alcohol, Loeffler’s methylene blue, oil immersion and microscope.
The process includes the following steps:
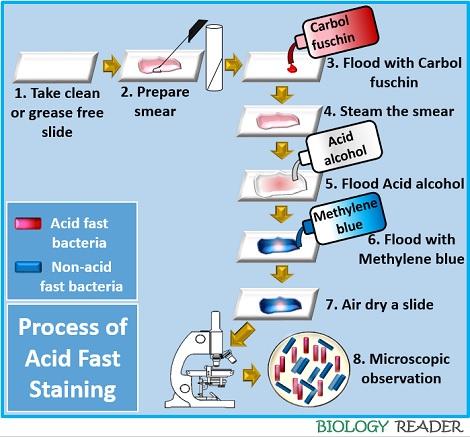
- Take a clean, sterilized slide.
- Then, add a drop of distilled water to the centre of the glass slide. After doing this, thoroughly mix the inoculum by a sterilized inoculating loop. Then, heat fix and air dry the prepared smear.
- Flood the smear with the primary stain (Carbol fuschin) and allow it to stand for one minute.
- Then, flood the smear with the decolourizer (3% HCl) and wash the glass slide with distilled water.
- Finally, flood the smear with the counterstain (Loeffler’s methylene blue) and allow it to stand for one minute. Again rinse the slide in water.
- Air-dry the glass slide.
- Observe the glass slide under oil immersion at 100X objective.
Observation
After performing acid-fast staining, we could observe the following changes, as you could see in the figure below.
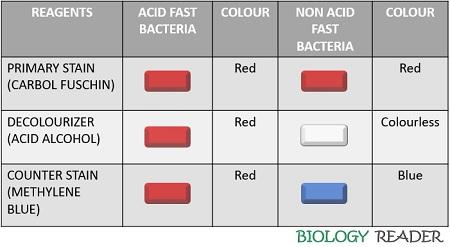 Without staining, the bacteria appear colourless. So, it becomes necessary to stain the bacteria to identify and classify them based on their physical and chemical differences. On primary staining, the ZNCF stains both the acid-fast bacteria and non-acid fast bacteria to appear red.
Without staining, the bacteria appear colourless. So, it becomes necessary to stain the bacteria to identify and classify them based on their physical and chemical differences. On primary staining, the ZNCF stains both the acid-fast bacteria and non-acid fast bacteria to appear red.
In the decolourization stage, non-acid fast bacteria lose the colour of the primary stain or appear colourless, whereas acid-fast bacteria appear red. Finally, the colourless non-acid fast bacterial cells take up the colour of methylene blue and appear blue. Oppositely, the acid-fast bacteria will remain red even after the counterstaining.
Interpretation of Result
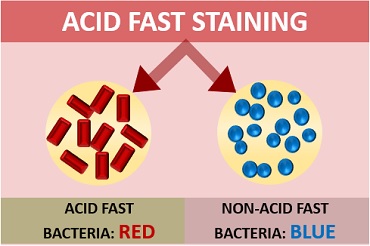
Acid-fast cells: They appear red coloured, rod shaped and can occur singly or in small groups.
Non-acid fast cells: They appear blue coloured.