Endospore staining is a type of special staining method, which makes use of differential staining procedure to highlight the presence or absence of endospores. For the staining of endospores, the first article was published by Dorner in the year 1922.
Later, the Dorner method of staining endospores has been modified by two scientists, Schaeffar and Fulton, in the year 1933. Schaeffer and Fulton had introduced a faster process to stain endospores where they used differential stain to highlight the bacterial endospore selectively.
The main objective of endospore staining is to differentiate the bacterial endospore from the vegetative cell, which allows us to distinguish between the endospore formers and non-endospore formers.
Content: Endospore Staining
- Definition of Endospore Staining
- Definition of Endospore
- Types of Endospore
- Methods of Endospore Staining
- Significance
Definition of Endospore Staining
Endospore staining is a unique staining method, which discriminates between the two major groups of bacteria (spore formers and non-spore formers) by selectively staining the endospore against the vegetative cell. Clostridium perfringes, Bacillus cereus, Sporosarcina sp etc., are examples of endospore formers. Escherichia coli, Salmonella sp etc., are examples of non-endospore formers. Endospores can be stained by employing the two methods, namely Dorner’s method and Schaeffar and Fulton’s method.
Definition of Endospore
These are the structures that form within the cell. It is the unique structure of the bacteria, which forms during the unfavourable conditions approximately within 6-8 hours. The endospores are thick, dormant, metabolically inactive, and the refractile bodies produced by the few genera of the bacteria like Bacillus and Clostridium species.
Endospores can remain in a resting phase or dormant state before they germinate into new individuals. They are resistant to extreme physical conditions (like radiation, heat and desiccation, etc.) and chemical conditions (like staining, disinfection, etc.). They can resist the boiling temperature for a few hours and heat treatment of 80 degrees Celsius for 10 minutes.
Endospores consist of a colossal amount of DPA (Dipiclonic acid), which constitutes about 10-15% of the spore’s dry weight. DPA resides within the endospore core and exists in combination with a large amount of calcium (Ca-DPA complex). The Ca-DPA complex provides heat resistance to an endospore. During germination, endospore loose resistance towards heat and staining.
Types of Endospore
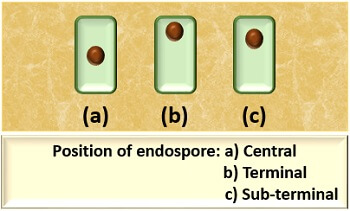
The position of endospore is different in different bacterial species, but commonly they exist in the following three types:
- Central: The location of an endospore is towards the centre of the vegetative cell.
- Terminal: The location of an endospore is towards an edge of the vegetative cell.
- Sub-terminal: The location of an endospore is between the central and terminal position of the vegetative cell.
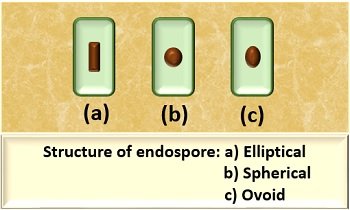
The structure of endospore also varies from individual to individual bacterial cell, but commonly they are of three types:
- Elliptical: An endospore appears long or elongated.
- Spherical: The endospore appears small and pear-shaped.
- Ovoid: An endospore appears small and egg-shaped.
Methods of Endospore Staining
We can practice endospore staining by employing one of the two methods given in the figure below.
The process of endospore staining follows a general staining procedure and uses three kinds of stain. A primary stain helps in the staining of the endospore.

Schaeffar and Fulton’s Method
It is the most common endospore staining method, which uses three reagents (primary stain, decolourizer and counterstain).
- Malachite green (primary stain): To prepare 0.5% of an aqueous solution, you need to take 0.5 g of malachite green in 100 ml of distilled water.
- Distilled water works as a decolourizer.
- Safranin (counterstain): To prepare 2.5% of an aqueous solution, you should take 2.5 g of safranin into 100 ml of 95% ethanol.
Procedure
Schaeffar and Fulton’s method includes the following steps:
- Sterilization of glass slide: Take a clean and sterilized glass slide.
- Smear preparation: Prepare smear by taking little bacterial culture over the glass slide using an inoculating loop.
- Heat fixing: Then, you need to fix the smear by moving the glass slide to and fro over the Bunsen burner’s flame.
- Saturation with primary stain: Place a blotting paper corresponding to a glass slide’s size and saturate it with malachite green dye.
- Steaming: Steam the saturated blotting paper for up to 5 minutes. During this step, you can add more dye as per the requirements.
- Washing: Rinse the glass slide in water.
- Counterstaining: Flood counterstain (safranin) over the glass slide and allow it to stand for 1 min and then wash off the slide.
- Air drying: After counterstaining, allow the glass slide to air dry.
- Microscopic observation: Add a drop of oil immersion on the stained area of a glass slide and observe it under the microscope of a 100X objective.
Principle
In Schaeffar and Fulton method, an endospore is subjected to heat as it cannot be stained directly, unlike a vegetative cell. The heat treatment allows the penetration of the primary stain, i.e. Malachite green, into an endospore’s cortex. Once they take up the dye, they retain the dye’s colour or become resistant to de-staining.
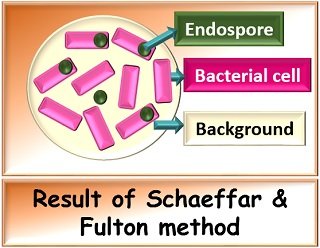
Result
Endospores retain the colour of the primary stain (malachite green) or appear green in colour. In contrast, vegetative cells lose up the primary stain and take up the counterstain colour (safranin) or appear pink in colour.
Dorner’s Method
It is an old endospore staining method, which uses three reagents (primary stain, decolourizer and counterstain).
- Carbol fuschin (primary stain): Take 0.3 g of basic fuschin, 10ml of ethanol (95%) and 95 ml of distilled water to prepare its solution.
- Acid alcohol (decolourizer): Take 97 ml of 95% ethanol in 3 ml of concentrated hydrochloric acid to prepare the decolouring agent.
- Nigrosine (counterstain): Take 10 ml of nigrosine in 100 ml of distilled water to prepare its solution.
Procedure
Dorner’s method includes the following steps:
- Sterilization of glass slide: Take a clean and sterilized glass slide.
- Smear preparation: Prepare smear by taking little bacterial culture over the glass slide using an inoculating loop.
- Heat fixing: Then, move the prepared slide to and fro over the Bunsen burner’s flame.
- Saturation with primary stain: Place a blotting paper corresponding to a glass slide’s size and saturate it with carbol fuschin dye.
- Steaming: Steam the saturated blotting paper for up to 5-10 minutes.
- Decolourizing: To decolourize the glass slide, add acid alcohol dropwise.
- Counterstaining: Place a drop of nigrosine on one end of the glass slide and drag another slide to make a thin film of stain. Allow the dye to stand for 1 min, and then wash off the slide.
- Air drying: After counterstaining, allow the glass slide to air dry.
- Microscopic observation: Add a drop of oil immersion on the stained area of a glass slide and observe it under the microscope of a 100X objective.
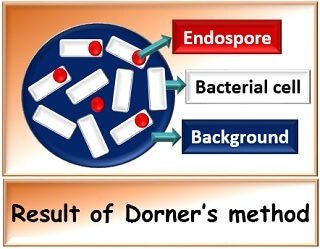
Result
Endospores retain the colour of primary stain, i.e. Carbol fuschin and appears red coloured, whereas vegetative cells appear colourless against a dark background stained with nigrosine.
Significance
Endospore staining helps in the microscopic study of the endospore, by which we can differentiate and classify the bacteria. It also plays a significant role in the medical science and food industry as the spores can be present in canned food, which results in food spoilage and ultimately cause food diseases in humans and animals.