The bile solubility test is a biochemical test that distinguishes bile soluble and bile resistant α-haemolytic Streptococci. Streptococcus pneumoniae is the only strain that emulsifies by reacting with the bile solubility reagent, whereas the other α-haemolytic Streptococci do not undergo such reaction.
The reason for the dissolution of the Streptococcus pneumoniae is due to the presence of the autolytic enzyme (Autolysins). In action to the bile spot reagent, the autolysins become activated. The autolysin amidases cause autolysis of bacterial cells by disintegrating the peptidoglycan backbone.
An intracellular autolytic amidase gets activated in response to a surface reagent like bile salts and cleaves the bond between an alanine and muramic acid of peptidoglycan. In this context, we will discuss the definition, principle, reagent preparation and three common methods of the bile solubility test.
Content: Bile Solubility Test
Definition
The bile solubility test is the qualitative method that primarily isolates the bile sensitive Streptococcus pneumoniae and bile resistant α-haemolytic Streptococci. It depends on the stimulation of autolysin amidase by the bile salt reagent. Streptococcus pneumoniae undergoes cell-lysis by the action of bile salt reagent and called “Bile soluble organisms”.
It involves direct and indirect method, in which the bile salt reagent can be added directly over the isolated test organism and indirectly added to the medium containing saline and heavy suspension of the test specimen.
Principle of Bile Solubility Test
The mechanism of the bile solubility test depends upon the “Autolysis reaction”. Streptococcus pneumoniae differs from the other alpha-haemolytic streptococci by giving favourable bile solubility and Optochin susceptibility test.
The bile solubility test relies upon the activity of cell-bound autolysin amidase Lyt-A enzyme (capable of lysing the cell). The enzyme becomes active in reaction to the surface-active reagents like bile salts.
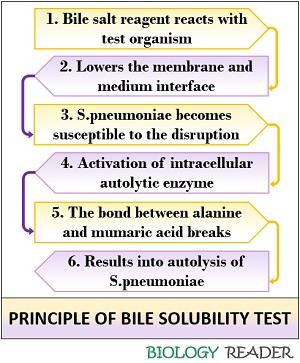
Bile solubility test makes the use of 2% and 10% bile spot reagent of sodium deoxycholate solution. The sodium deoxycholate solution reagent carries out the sequential degradation of the bacterial cell by first lowering the surface tension between the medium and then the cell membrane interface.
Bile salts result in the cell disruption of Streptococcus pneumoniae, after which an intracellular autolytic enzyme activates. This enzyme will break the bond between an alanine and muramic acid of the peptidoglycan and finally cause autolysis of the bacterial cell.
Bile solubility reagent
It is prepared by dissolving bile salt like sodium deoxycholate in sterile water.
- To prepare 2% bile solubility reagent: Add 2 grams of sodium deoxycholate bile salt in 100 ml of sterile water.
- To prepare 10% bile solubility reagent: Add 10 grams of sodium deoxycholate bile salt in 100 ml of sterile water.
Bile Solubility Test Procedure
The bile solubility test includes the following three methods.
Tube Method
It is an indirect method, which involves the following steps:
- Take sterile test tubes and pour 2 ml of 0.85% saline solution.
- Then, inoculate heavy inoculum from the blood agar via a sterile inoculating loop.
- Thoroughly mix the bacterial suspension with the saline solution.
- Then, equally part the solution containing saline and bacterial suspension into two test tubes and label them as control and test culture. The turbidity of both the test tubes should be between 0.5-1.
- In a test culture tube, add two drops of bile salt reagent, whereas in the control tube, adds two drops of saline.
- Vigorously shake the test tubes to ensure uniform mixing of the cell suspension with the reagent.
- After that, incubate the tubes for 18-24 hours at 35 degrees Celsius.
- At last, observe the tubes for the turbidity clearance.
Test Results
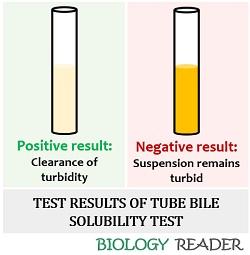
- Positive result: Turbidity disappears.
- Negative result: Here, the suspension remains turbid.
Plate Method
It is a direct method, which involves the following steps:
- Add two drops of 10% bile salt reagent over the test isolate in the blood agar media.
- Mix the reagent by rotating the Petri plate back and forth.
- Then, subject the test plates to an incubator at 35-37 degrees Celsius for a maximum of 30 minutes.
- Examine the plates for the appearance of a disintegrated colony.
Test Results

- Positive result: Disintegrated colonies appear after 30 minutes.
- Negative result: Unlysed colonies indicates a negative result.
Slide Method
It is also a direct method to perform the bile solubility test, which includes the following steps:
- Add one drop of bacterial suspension over the clean, grease-free glass slide.
- Then, add a drop of bile spot reagent on a glass slide.
- After that, air dry the glass slide.
- At last, perform the gram staining procedure.
Test Results

- Positive result: Cocci will not appear under microscopic examination.
- Negative result: Cocci appears under the microscopic examination.
Limitations
The bile solubility test includes the following limitations:
- The bile solubility test only examines the presence of Streptococcus pneumoniae among the other alpha haemolytic Streptococci.
- If a test organism undergoes incomplete or partial autolysis, the bile solubility test provides a possible way to determine the presence or absence of pathogenic strain Streptococcus pneumoniae. But confirmed after practising the “Optochin susceptibility test”.
- The higher concentration of bile salt may also restrain the autolysis of Streptococcus pneumoniae.
- It is unreliable to test the old bacterial culture, as it may lose the metabolically active enzyme, and the saline and broth should have a neutral pH-7.
Conclusion
Therefore, we can conclude that the bile solubility test helps in the identification and differentiation of Streptococcus pneumoniae from other Streptococci, based on the property of bile solubility. S.pneumoniae is bile sensitive, as it emulsifies by the reaction of bile salt reagent via an intracellular autolytic enzyme, whereas other strains are bile resistant.