The difference between osmotic pressure and osmotic potential is characterized by the properties like a chemical equation, effect of solute particles and types of system where it develops.
The chemical equation for the osmotic pressure is equal to the product of molar concentration, gas constant and temperature. In contrast, the chemical equation for the osmotic potential is a product always equal to the factor of solute concentration, gas constant and temperature.
A high concentration of solutes increases the osmotic pressure, and thereby its value for a solution is always positive. Oppositely, a high concentration of solutes decrease the osmotic potential, and thereby its value for a solution is always negative.
Osmotic pressure requires a confined or closed system, whereas solute potential may develop in both the open and confined systems. This post describes the key differences between the terms osmotic pressure and osmotic potential, along with the comparison chart. You will also get to know the definition, formula and similarities between the two.
Content: Osmotic Pressure Vs Osmotic Potential
Comparison Chart
| Properties | Osmotic Pressure | Osmotic Potential |
|---|---|---|
| Meaning | It refers to the physical pressure exerted into a solution when separated from pure water through a semipermeable barrier | It refers to the potential of solute particles that lower the water potential or free energy of water molecules within a solution |
| Denoted as | π | Ψ |
| Formula | π =MiRT | Ψs=CRT |
| Addition of solutes | Increases the osmotic pressure | Decreases the osmotic potential |
| Value for a solution | Positive | Negative |
| Requirement | It develops in a confined space | May develop in a confined as well as the open system |
| Occurs due to | Movement of free water molecules | Movement of dissolved solutes |
| Proportional to | Directly proportional to the molar concentration | Directly proportional to the solute concentration |
| Effect of solute particles | Its value increases by the increasing concentration of solute particles | Its value decreases by the increasing concentration of solute particles |
| Results in | Reduces the diffusion pressure of water in a solution or cell | Reduces the water potential by decreasing the kinetic motion of free water molecules |
Definition of Osmotic Pressure
It refers to the pressure difference between the solution and pure solvent resulted after adding solutes to one side. Osmotic pressure can also be defined as the pressure applied to a solution in order to nullify osmotic flow. It is a colligative property, which means that solute particles in a solution directly influence the osmotic pressure.
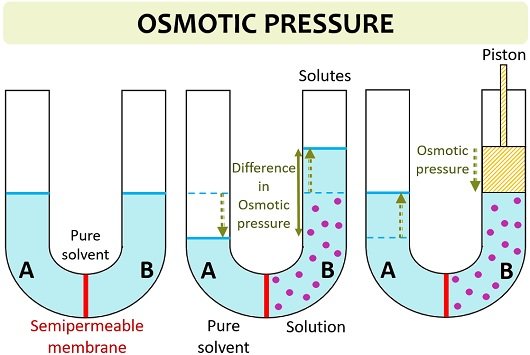
More solute concentration will cause the movement of pure solvent towards the side of high solute or low water concentration, thereby causing a potential difference between the two sides.
Formula
Osmotic pressure (π) = MiRT
- M= Molar concentration or the number or mole of solutes dissolved in ‘V’ litres of solution, i.e. mol/L.
- i= Van’t Hoff’s factor (It is dimensionless)
- R= Gas constant ((8.3144598 J.mol-1.K-1)
- T= Absolute temperature in Kelvin (K)
Definition of Osmotic Potential
It refers to the potential of solutes that facilitate the movement of water from a hypotonic region towards the hypertonic state (contains less water and more solutes). Osmotic potential is also known as solute potential, and it lowers the chemical potential or free energy of pure water due to solute particles.
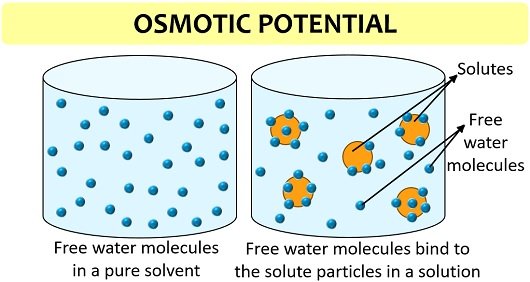
It is also a colligative property, i.e. depends upon the concentration of solutes in the solution. More solute concentration will decrease the random motion of the water molecules, thereby decreasing the water’s chemical potential.
Formula
Solute potential (Ψs) = CRT
- C= Concentration of solutes
- R= Universal gas constant (i.e. 8.3144598 J.mol-1.K-1)
- T= Absolute temperature in Kelvin (K)
Key Differences Between Osmotic Pressure and Osmotic Potential
- Osmotic pressure (π) is the physical pressure exerted into a solution when separated from pure water through a semipermeable barrier. Osmotic or solute potential (Ψs) refers to the potential of solute particles that lower the water potential or free energy of water molecules within a solution.
- The addition of more solutes increase the value of osmotic pressure and decreases the value of osmotic potential. Thus, the value of osmotic pressure for a solution is positive, whereas negative for the solute potential.
- Osmotic pressure develops in a confined space (does not allow an exchange of matter). Oppositely, the solute potential may develop in a closed as well as the open system (enables an exchange of matter with the surrounding).
- The value of osmotic pressure increases by the increasing concentration of solute particles, as it is directly proportional to the molar concentration of solutes in a solution. Conversely, the osmotic potential is directly proportional to the solute concentration, so its value decreases by the increasing concentration of solute particles.
- Osmotic pressure reduces the diffusion pressure of water in a solution or cell. In contrast, solute potential lowers the water potential by decreasing the kinetic motion of free water molecules.
Similarities
- Osmotic pressure and solute potential is a colligative property. Both depend upon the concentration (not on the type) of solute particles and the type and amount of solvent.
- The value of osmotic pressure and solute potential for pure solvent is always maximum, i.e. zero.
Conclusion
The difference between the osmotic pressure of solutions develops thirst of the cell or diffusion pressure deficit, and an increase in DPD increases osmotic pressure. As a result, endosmosis or subsequent reduction in the diffusion pressure of water occurs in a solution. It is resulted due to solute molecules and forces opposing diffusion.
A high concentration of solutes lowers the osmotic potential, as adding solute particles into a pure solvent gradually decreases the chemical potential or free energy of water. Thus, free water molecules’ kinetic energy or random motion in a pure solvent decreases to some extent as free water molecules bind with solute molecules. So, the solute potential decreases the water potential.