Thymine and Uracil are the two nucleotide bases which are found in the DNA and RNA respectively. The main difference between thymine and uracil is due to the property of “Occurrence”. The occurrence of both thymine and uracil is the property that distinguishes these two, as thymine is a pyrimidine nitrogenous base found in deoxyribonucleic acid (DNA). In contrast, Uracil is a pyrimidine nitrogenous base found in ribonucleic acid (RNA).
A deoxyribonucleic acid possesses cytosine and thymine as the pyrimidine bases, while a ribonucleic acid contains cytosine and uracil as the pyrimidine bases. You will get to know the key differences and similarities between the thymine and uracil nitrogenous base along with the comparison chart. Besides, we will also discuss the definition, structure, and important facts about both thymine and uracil.
Content: Thymine Vs Uracil
Comparison Chart
| Properties | Thymine | Uracil |
|---|---|---|
| Uracil | It is the pyrimidine base of the DNA | It is the pyrimidine base of the RNA |
| Occurrence | Found only in DNA | Found only in RNA |
| Presence of keto group | Two keto groups present at C-2 and C-4 atom | Two keto groups present at C-2 and C-4 atom |
| Presence of methyl group | One methyl group present at C-5 atom | Not present |
| Molecular formula | C5H6N2O2 | C4H4N2O2 |
| Molar mass | 126.1133 g/mol | 112.0868 g/mol |
Definition of Thymine
The nitrogenous base comes under the type of pyrimidine base, which appears as a single ring structure within the backbone of deoxyribonucleic acid where it complements pairs with the purine base adenine by two hydrogen bonds. A methyl group is linked to the 5′-C of the thymine’s aromatic ring structure. It was first reported by the two scientists named Albrecht Kossel and Albert Neumann. Thymine was named after its presence in the calve’s thymus gland. Some of the chemical properties are mentioned below:
- Chemical formula: C5H6N2O2
- Molar mass: 126.115 g/mol-1
- Density: 1.223 g/cm-3
- Melting point: 316-317 degrees Celsius
Definition of Uracil
The nitrogenous base comes under the type of pyrimidine base, which appears as a single ring structure within the backbone of ribonucleic acid where it complements pairs with the purine base adenine by two hydrogen bonds. A hydrogen atom is linked to the 5′-C of the uracil’s aromatic ring structure. It was first reported by a scientist named Alberto Ascoli. Uracil was first derived by the hydrolysis of yeast nuclein. Some of the chemical properties are mentioned below:
- Chemical formula: C4H4N2O2
- Molar mass: 112.08 g/mol1
- Density: 1.32 g/cm3
- Melting point: 335 degrees Celsius
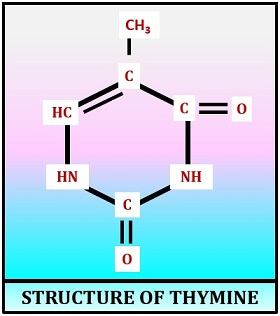
Structure of Thymine
Thymine is the pyrimidine base of the DNA, which contains two keto groups at C-2 and C-4 position and one methyl group at the C-5 position. It is denoted as T. It is synthesized by uracil methylation at the C-5 position of the pyrimidine ring, due to which thymine is also called 5-Methyl uracil. In DNA helix, the complementary pair of thymine is the purine base (adenine). The nitrogenous bases T and A pairs with each other by forming two hydrogen bonds.
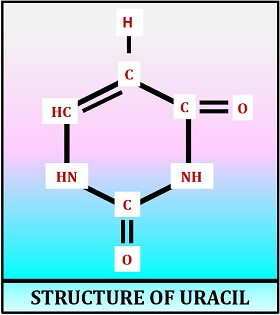
Structure of Uracil
Uracil is the pyrimidine base of the RNA, which contains two keto groups at C-2 and C-4 position. It is denoted as U. In an RNA molecule, the complementary pair of uracil is the purine base (adenine). The nitrogenous bases U and A pairs with each other by the formation of two hydrogen bonds.
Important Facts
As we know that the combination of the nitrogenous bases and pentose sugar forms a nucleoside. The attachment of one or more phosphate groups to the nucleoside forms a nucleotide.
- Therefore, the combination of thymine with DNA pentose sugar will form deoxythymidine.
- Same goes with uracil, i.e. it will form uridine by combining with RNA pentose sugar.
- Now coming onto the concept of nucleotide, the combination of deoxythymidine with the monophosphate group will constitute deoxythymidine-5’ monophosphate (5’-dTMP). Similarly, the attachment of diphosphate and triphosphate groups to the deoxythymidine will form deoxythymidine-5’ diphosphate (5’-dTDP) and deoxythymidine-5’triphosphate” (5’-dTTP), respectively.
- The pairing of uridine with a monophosphate group will constitute uridine-5’monophosphate (5’-dUMP). Similarly, the diphosphate and triphosphate group’s attachment to the uridine complex will form uridine -5’ diphosphate (5’-dUDP) and uridine -5’triphosphate (5’-dUTP), respectively.
Key Differences Between Thymine and Uracil
- Thymine is the pyrimidine base of the DNA, whereas Uracil is the pyrimidine base of the RNA.
- Methyl group is absent in uracil, while present in thymine at the C-5 position.
- The molecular formula of thymine is C5H6N2O2, whereas the molecular formula of uracil is C4H4N2O2.
- Thymine has a molar mass of 126.1133 g/mol and uracil has a molar mass of 112.0868 g/mol.
Similarities
- Both thymine and uracil are the nitrogenous bases present in the nucleic acid, i.e. DNA and RNA.
- Thymine and uracil are the two pyrimidine nitrogenous bases.
- Both complementary pairs with the purine base, i.e.“Adenine”.
- Both can form a dimer in the presence of ultraviolet light.
Conclusion
Therefore, we can conclude that thymine and uracil are the two pyrimidine nitrogenous bases with the different molecular structure, molecular formula, molecular weight and occurrence. Inspite of many differences, there are similarities like the type of nitrogenous base and their complementary base pair. So, we can say thymine and uracil bases are the component of the genetic material, i.e. DNA and RNA, respectively.