Endosmosis is the diffusion of water from the cell exterior to the cell interior or cytoplasm. The study of endosmosis is possible if you keep a cell in the hypotonic solution.
In endosmosis, the solute and solvent concentration inside and outside the cell are as follows:
- High solvent and low solute concentration in the cell surrounding than in the cell interior.
- High solute and low solvent concentration in the cell cytoplasm than in the cell surrounding.
Cells in hypotonic solution have a high solute concentration than outside. But according to the rule of osmosis, solutes cannot cross the selective barrier, only water can.
For this reason, the water from its high concentration, i.e. cell surrounding will enter the cell cytoplasm crossing a selective barrier.
Endosmosis makes the cell swell, turgid or even burst. In this post, we will discuss the definition of endosmosis and some examples to illustrate the mechanism.
Content: Endosmosis Examples
- Definition
- Swelling of Raisins in Pure Water
- Endosmosis in Plants
- RBCs Swell in Hypotonic Solution
- Pruning of Fingers
- Thirst Developed due to Eating Salty Food
- Endosmosis in Fish
- Absorption of Water by Intestine
- Conclusion
Definition of Endosmosis
Endosmosis refers to water movement from the cell surrounding (with high water potential) to the cell (with low water potential). In other words, endosmosis is the diffusion of water into the cell. As a result, cells in the solution swell, become turgid or may get ruptured sometimes.
Let us discuss some real-life examples to study the mechanism of endosmosis.
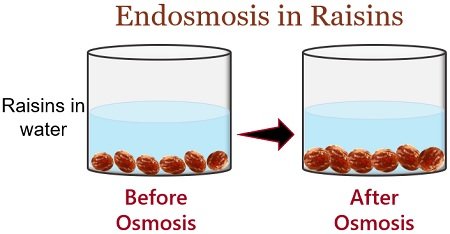
Swelling of Raisins in Pure Water
You can perform this activity at your home by simply taking any container, water and some raisins. In the diagram below, you can see a change in raisins before and after osmosis.
To see the results, follow the given protocol:
- First, take a beaker or any container.
- After that, add a few raisins into the beaker.
- Then, add water to half of the beaker.
- Then you have to wait for a while to conclude the result.
After performing this activity, you could see the swelled raisins. Now the questions are, what happened during the osmosis, and why do raisins get swelled?
Well, raisin cells absorb water during osmosis. And the swelling of raisins occurs due to:
- The high solvent concentration in the surrounding solution.
- The cell interior of raisins contains less water and more solutes like sugar.
Thus following osmosis, water travels from the cell surrounding to the interior of raisins.
Endosmosis in Plants
Plants absorb water through leaves, stems, and roots. But, the majority of the water absorption in plants is by roots. Roots absorb water from the soil via endosmosis.
Tiny root hairs or absorbent hairs in the maturation zone of the root absorb water from the soil. They exist as single-celled extensions of epidermal cells in the root.
The cell membrane of root hair cells acts as a semipermeable barrier. It allows solvent (water) to move from the high concentration region (soil) to the low concentration region (roots).
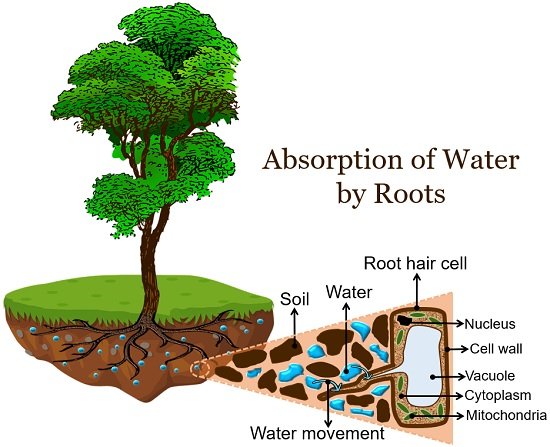
Consequently, root hair cells become turgid and their osmotic pressure or the tendency to take in solvents decreases.
Root hair cells exert pressure on adjacent cells, after which water molecules enter xylem tubes. This pressure is known as root pressure.
Further, xylem tubes conduct water to other plant parts via capillary action. In this way, root hairs absorb water incorporated within the soil through endosmosis.
RBCs Swell in Hypotonic Solution
Red blood cells are small, red coloured biconcave discs that give the blood its characteristic colour. The red blood cells have a semipermeable cell membrane.
We could see swelling of RBCs when they are placed in a freshwater or hypotonic solution. RBCs in hypotonic solution have a high concentration of ions and solutes and a low solvent concentration than the surrounding.
As per the rule of osmosis, water moves from the high solvent concentration to the low solvent concentration.
Thus, the water surrounding RBCs travels into the cytoplasm of RBCs via endosmosis. It causes swelling of red blood cells.
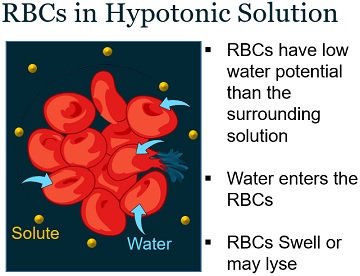
The turgor pressure exerted on the cell membrane control the amount of water that can travel into the RBCs.
Sometimes, RBCs consume more water than their membrane can handle. It leads to the lysis of red blood cells, called hemolysis.
Pruning of Fingers
Sometimes you must have seen your fingers pruned after submerging them in water for a while. Pruning is mainly seen in tough skin like the foot and palm.
The cell membrane around the skin cells is not a perfect seal and permits water to move into or out of cells.
Pruning occurs when the cytoplasm of skin cells has a high concentration of salty water. In this case, if you put your fingers into regular or freshwater, the water will flow into the skin cells.
As a result, cells in the fingers absorb water and get expanded or bloated. It results in the pruning of fingers. Sometimes, it’s quite strange to see wrinkled or pruned digits.

But, it is believed that the pruning of fingers is due to endosmosis. Thus, water enters the skin cells through the semipermeable cell membrane, causing them to swell and wrinkle.
Thirst Developed due to Eating Salty Food
After eating salty foods, you probably feel thirsty, and you would likely search for water. So, after consuming lots of salt:
- Our cells become concentrated with salt.
- The concentration of salts and minerals in your blood increases.
The above two trigger the process of thirst or inducing osmotic thirst, so we start drinking water.
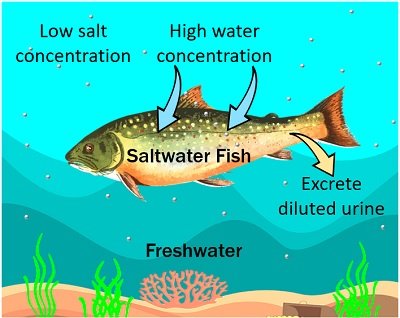
Endosmosis in Fish
The mechanism of water absorption in fishes is through their skin and gills. Through osmosis, they regulate the concentration of their body fluids.
Freshwater is hypotonic for the saltwater fishes that are living in it. The body fluids of saltwater fish contain more concentration of salts than the salt content outside.
As a result of osmosis, there will be constant water flow into the body of saltwater fishes. So, the saltwater fish urinate frequently to survive in this continuous water supply but cannot survive longer.
Absorption of Water by Intestine
Osmosis occurs when the chyme from the stomach reaches the small intestine. Intestinal epithelial cells contribute to the lining of the intestines.
Intestinal epithelial cells have low solvent concentration than in the chyme. Thus following osmosis, water moves into the intestinal cells through a semipermeable membrane.
Later, the water moves into the bloodstream via the capillaries near the epithelial cells.
Conclusion
Thus, endosmosis is a type of osmosis in which the water moves into the cell from the surrounding.
In endosmosis, the cell contains more solutes and less solvent concentration. In contrast, the cell surrounding contains low solute and more solvent concentration.
The water movement into the cell is due to the difference in the concentration gradient between cells and surroundings.

Nice article.