Do you know the examples of adsorption? Adsorption is a common term that we must have heard many times. In adsorption, gas or solute particles adhere to the surface interface of the solid or liquid matrix. So, it is a surface phenomenon that works opposite the absorption mechanism.
The gas or solute molecules that adhere to the surface of any matrix are called adsorbates. In contrast, the adsorbent is a solid or liquid matrix upon which the gas or solute particles stick.
Activated alumina, silica gel, activated carbon, zeolites etc., are good adsorbents. These have highly porous structures and hence a large surface area for adsorption.
Dirt or impurities in the raw water coagulate around the alum molecule is a classic example of adsorption. Here, the impurities in the raw water function as adsorbates and alum behave as an adsorbent. In adsorption, a molecular or atomic layer of adsorbate forms on the adsorbent surface.
A single or multiple layers of adsorbate could be formed on the adsorbent. Adsorption is reversible, i.e. adsorbates can be separated from the adsorbent. This post overviews what adsorption is and explains examples of adsorption in daily life.
Content: Examples of Adsorption in Daily Life
What is Adsorption?
Adsorption is a surface phenomenon in which adsorbates remain at the surface of the adsorbent. Or, we can say that adsorbates do not penetrate into the bulk.
Adsorbate is the accumulated end product.
Adsorbent is the material used as an adsorption medium.
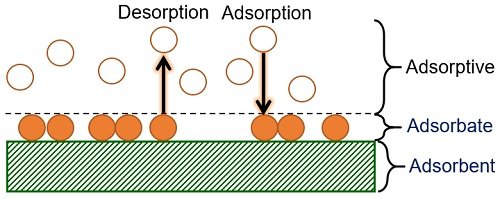
Associated Terms
- Sorption is a phenomenon involving both adsorption and absorption. Dipping sponge in water, dipping cotton in the dye etc., are examples of sorption.
- Desorption is the reverse of sorption or opposite of adsorption.
Principle
The molecules or atoms interacting with the adsorbent surface have residual surface energy. The energy generates due to the unbalanced forces that attract the molecules to stick onto the surface.
Based on the Adsorption Forces: Physisorption and Chemisorption are the two types of adsorption.
- Physical adsorption is gas accumulation on a solid or liquid surface via Vander Waal’s forces. Physical adsorption is nonselective and has a fast adsorption rate.
- Chemical adsorption involves gas or atoms accumulation on a solid or liquid surface via chemical bonding. The bonds could be covalent or ionic, which continuously form and destroy.
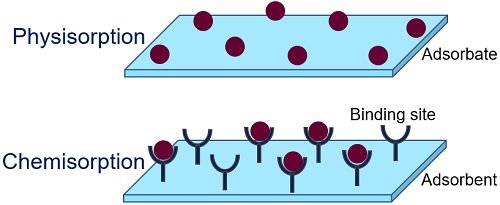
Nature of Reaction
Physical or chemical bonds hold the adsorbates on the adsorbent medium, releasing heat from the surface. So, adsorption is an exothermic reaction, i.e. ΔH is always negative.
Factors Affecting
The rate of the process depends largely on surface area and temperature.
- The adsorbent’s large surface area and small particle size offer greater adsorption at desired temperature and pressure.
- Low temperature promotes adsorption. At low thermal energy, molecules will have low kinetic energy. Due to this, molecules are likelier to stick to surfaces via covalent or hydrogen bonds.
Examples of Adsorption
Adsorption is helpful in water purification, sugar purification, pollution control, trapping microorganisms and many other things. Examples of adsorption include the following:
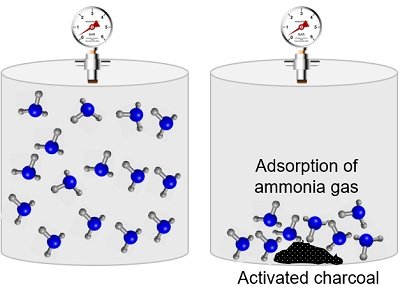
Adsorption of a Gas by Activated Charcoal
A piece of activated charcoal can adsorb gases like ammonia, oxygen, hydrogen, chlorine and others. So, charcoal acts as an adsorbent while gas molecules act as adsorbates.
You can perform one experiment by enclosing one of these gases in a closed vessel containing powdered charcoal. After some time, you will notice a decrease in the pressure of the gas inside the vessel. It is because the gas molecules adhere to the surface of the charcoal.
Adsorption of Ink on Chalk
Take any staining reagent or ink in a beaker and dip a chalk stick into that. Later, you will observe that the surface of the chalk stick will adsorb all the coloured molecules in ink. You will find a chalk white from inside if you break the chalk.
So, ink adsorption on chalk is a physisorption process in which the pigments adsorb on the chalk surface. One thing you should remember is that the solvent of ink absorbs inside the chalk.

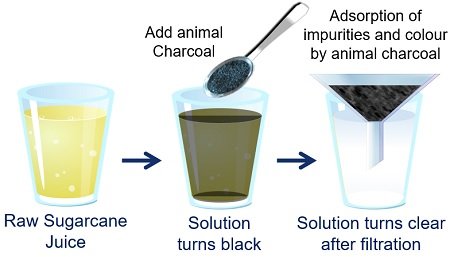
Animal Charcoal Adsorbs Impurities of Raw Sugar
Take sugarcane juice in a glass. After that, add one tablespoon of animal charcoal to the sugarcane solution and stir it well. Then, you will obtain a clear sugar solution by filtering the previous sample.
It occurs because animal charcoal acts as a decolorizing agent, allowing sugar to achieve its desirable white colour by adsorbing all the impurities and colouring matter of the sugarcane juice.
Silica Gel Adsorbs Air
The silica gel is a highly porous material with millions of voids and a surface area of around 800m2/g. It has a powerful adsorbing capacity for moisture or water vapours at a temperature near or higher than 40 degrees Celsius.
Silica gel is generally available as a solid, beaded or crystallized form of sodium silicate. The air becomes dry in the presence of silica gel because its tiny pores can adsorb and hold moisture.

The water vapours adhere to the surface of the silica gel, as seen in the above image. You must have seen the beaded silica gel in a small paper packet. Being a desiccant, it controls local humidity to prevent deterioration of some goods.
Gas Masks Adsorbs Poisonous Gases
Gas masks contain activated charcoal that adsorbs poisonous gases from the air. It involves physisorption where no chemical bonding occurs between the activated charcoal and gases. Instead, the gases and water vapours in the atmosphere stick to the surface of activated charcoal.
The adsorbent reacts multiple times with the oxygen to prevent the wearers from inhaling toxic gases like Co, CH4, etc. and air particulates. The air that passes the adsorbent is pure and safe to breathe. Thus, gas masks are good for purifying the air for breathing.

Paint Adsorption on Walls
Do you ever wonder how the paint sticks to the surface of the wall? Well, this is also due to adsorption. Paint industries employ adsorption techniques to remove the dissolved gases in the paint. Removal of dissolved gases is a must; otherwise, paints will not adhere properly to the walls.

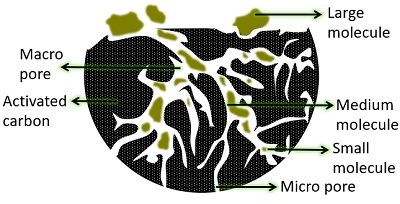
Adsorption of Contaminants by Activated Carbon Filters
Activated carbon filtration is a standard method in water treatment. It is designed to adsorb contaminants dissolved in water by pulling out or trapping them onto the surface of a filter.
Activated carbon filters efficiently reduce unwanted taste, odours, and micropollutants from the raw water. However, it can remove microbial and inorganic contaminants (metals, nitrates, etc.).
Conclusion
Thus, the adsorption phenomenon has a significant role in daily life, as it is largely used to remove undesirable colouring matter and impurities from various resources that we breathe and consume.