Lipid aggregates exist in varying forms starting from monolayer to structures like lamellar, tubular, cubic etc. The different structures or aggregates of lipids are known as phases. Largely, lipids are hydrophobic or water-insoluble.
Lipids assemble into microscopic aggregates in the aqueous environment because of their amphipathic nature. They possess both hydrophilic (a polar head) and hydrophobic moieties (a nonpolar tail). A polar head is constantly in touch with the aqueous environment.
Oppositely, a hydrophobic nonpolar tail does not interact with the surrounding water. Lipid aggregates form a phase or system separated from the surrounding aqueous phase. This post describes the definition, influencing factors and common types of lipid aggregates.
Content: Lipid Aggregates
Definition of Lipid Aggregates
They refer to the cluster of lipid molecules that assemble due to the association of hydrophobic moieties and the interaction of hydrophilic groups with the surrounding water. Lipids may form stable aggregates like micelle and thermodynamically unstable aggregates like bilayer sheets. The dissolution of amphipathic lipids in water causes the assembly of lipid molecules in different phases. Lipid aggregates have hydrophilic ends outside and hydrophobic ends inside under oil in water emulsion.
Factors Driving Lipid Aggregation
There are generally three factors responsible for clustering lipid molecules.
- The concentration of surfactant: It should be greater than the critical micelle concentration. Surfactant refers to the active material that facilitates the emulsion of a liquid upon entering. Critical micelle concentration or CMC refers to the concentration above surfactant that causes micelle formation.
- Krafft temperature: It refers to the temperature at which surfactants form lipid aggregates. Temperature below the Krafft temperature will not cause spontaneous rearrangement of lipid molecules in the aqueous environment.
- Hydrophobic effect: It facilitates aggregation of nonpolar solutes like lipid molecules from an aqueous environment.
Types of Lipid Aggregates
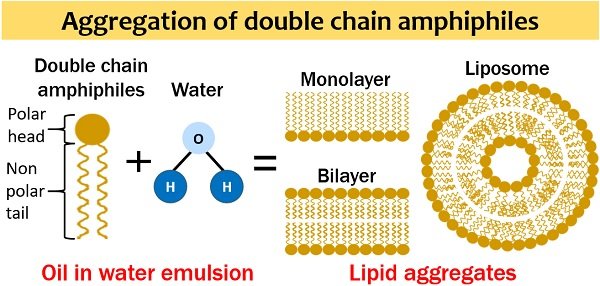
Lipids are generally single and double chain amphiphiles. Single chain amphiphiles form a monolayer and micelle. Oppositely, double chain amphiphiles form a bilayer and liposome. We can also observe some other structures, but these four are the most common.
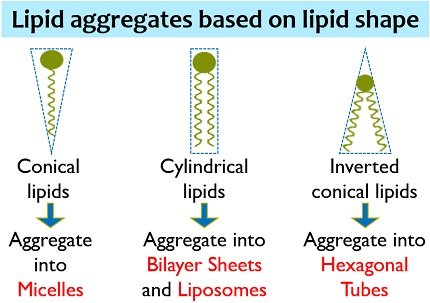
Non-covalent forces or some physical processes are responsible for the formation of lipid aggregates. Conical lipids like polyphosphoinositides and lysophospholipids self-aggregate into micelles.
Cylindrical lipids like sphingomyelin and phosphatidylcholine form flat bilayers. Inverted truncated lipid such as phosphatidylethanolamine forms hexagonal tubes.
Micelles
They appear relatively small and spherical. Size ranges between 2-20 nm. Several dozens to several thousand lipid molecules associate to form micelles. Ionized fatty acids form micelles. Hydrophobic moieties aggregate inwards, and hydrophilic moieties are in contact with the water.
This structure minimizes the polar-nonpolar interactions between the aqueous environment and hydrophobic tails. It allows the association of the polar head with each other and water and hides the hydrophobic tail inside. Micelles belong to the class of aggregation colloids as they exhibit colloidal state properties.
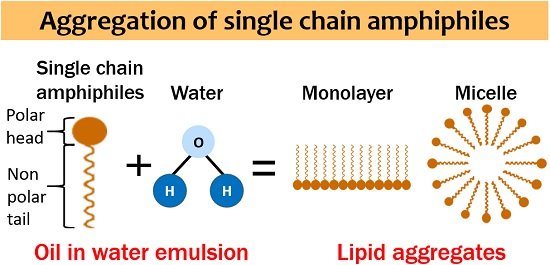
Lysophospholipids and SDS detergent form micelle in the aqueous environment. Oil in water emulsion is a standard method to demonstrate micelle formation where you can see a hydrophobic core and hydrophilic shell.
Micelles can also be formed in nonpolar organic solvents, after which you can observe the hydrophobic tails pointed outwards and hydrophilic heads pointed inwards. The process of micelle formation is called micellization.
Lipid Bilayer
The association of two lipid monolayers form a “Bimolecular sheet”. Double chain amphiphiles usually produce a bilayer sheet in an aqueous solution. The lipid bilayer generally exists as a two-dimensional sheet.
Here, the hydrophobic moieties of both the chains interact with each other. In contrast, the polar head groups or hydrophilic moieties interact with the surrounding water. A bilayer sheet has identical head groups and side chains. Two opposite layers are called leaflets.
Thus, the lipid bilayer serves as a barricade between both the membrane’s surfaces. Hydrophobic interactions like Vander Waals forces and electrostatic interactions stabilize the bilayer sheet.
Glycerophospholipids and sphingolipids are examples of the lipid bilayer. Bilayer sheet possesses a high propensity to fix or fold itself. The structure of bilayer sheets easily deforms as they are thermodynamically unstable.
Liposome
It forms once the lipid bilayer folds on itself. It seems like a hollow sphere. Due to the folding of a lipid bilayer, hydrophobic edges disappear. Its size ranges between 25 nm to 1 µm. Among other lipid aggregates, liposomes possess maximum stability in the aqueous environment.
Liposomes or lipid vesicles can enclose water as they possess a small hydrophilic core at the centre. Vesicles vary in diameter. They have small aqueous filled compartments. Multilamellar vesicles, small unilamellar liposome vesicles, large unilamellar vesicles, and cochleate vesicles are the four significant liposomes.
Conclusion
Lipid aggregates form due to the amphipathic nature of lipids. They have a hydrophobic region comprising nonpolar hydrocarbon chains that are water-insoluble. Also, the hydrophilic region comprises polar head groups that are water-soluble.
The polar head readily pairs up the water molecules via electrostatic interactions or hydrogen bonds. The property of lipids in having both hydrophobic and hydrophilic regions cause aggregation of lipids in the aqueous environment. Therefore, lipids show polymorphism by aggregating into different shapes or phases.
Solute-solvent ratio, temperature, pressure and ionic strength influence the structural phase of the aggregation. Hydrophobic interactions between lipid molecules serve as a significant driving force in forming and maintaining the configuration of lipid aggregates.