A motility test refers to the biochemical or microscopic examination of an organism that checks the existence of cellular motility. By performing this test, we could differentiate the two major groups of bacteria, namely motile and non-motile, based on their cellular movement.
Few organisms are motile, and some are non-motile. Still, all living organisms tend to show a certain kind of movement in search of food against environmental stresses, for which locomotion is a common phenomenon.
Organisms locomote through various means; some show flagellar movement, while some show non-flagellar locomotion. This post describes the definition, purpose, principle, test media and two common procedures of motility test (slide and tube test) along with its uses and limitations.
Contents: Motility Test
Motility Test Definition
The motility test is the analytical method, which examines whether the bacteria are motile or non-motile through biochemical and microscopic analysis. Tube test is a biochemical method giving macroscopic and cumulative results after reducing the biological media (triphenyl tetrazolium chloride) by the motile organisms.
Slide test is a microscopic analysis of microorganisms, which involves direct examination (based on the cell’s microscopic features) by employing two popular methods (hanging drop and wet mount slide test).
Motility Test Purpose
- The motility test aims to check the cellular motility of a test organism.
- It provides a valuable tool to determine an organism’s ability to locomote within the liquid or semi-solid media.
- The motility test also distinguishes bacteria based on their motility.
Example: Like Yersinia, enterocolitica tends to be motile at a temperature of 20-25 degrees Celsius. Capnocytophyta is the bacterium that moves via gliding motility, whereas few bacteria like Campylobacter species are non-motile.
Principle of Motility Test
The motility test depends upon the organism’s tendency to move either in liquid or semisolid media. Eukaryotes locomote through the flagella, cilia, pseudopodia, whereas prokaryotes locomote through the propeller-like flagella and sometimes glide through their protein fibrils.
Bacteria also show chemotaxis property by surrounding themselves near to chemical compounds like sugar, amino acid etc. Motility test makes the use of triphenyl tetrazolium chloride media, which in oxidized form remains colourless.
When the bacteria grow in the triphenyl tetrazolium chloride media, they absorb the dye by causing a reduction of the media. In a reduced state, the bacterial growth is indicated by forming an insoluble red coloured complex (Formazon). An organism’s motility is detected by the immense red colour formation around the line of inoculation.
Test Media
Motility test requires the use of a standard media like TTC (triphenyl tetrazolium chloride) that contains the following ingredients:
- Beef extract: 3 grams
- Pancreatic digest of casein: 10 grams
- Sodium chloride: 5 grams
- Agar: 4 grams
- Distilled water: 1 litre
TTC is the most popular media to check whether a test organism is motile or non-motile. It is also available as pre-poured tubes by some of the biological company suppliers. The motility of an organism can also be tested by the use of multitest media like (MIL) motility indole lysine, (MIO) motility indole ornithine, (SIM) sulfide indole motility test, and mannitol motility media.
Motility Test Procedure
There are two standard methods to perform motility test:
Tube Test
It involves the following steps:
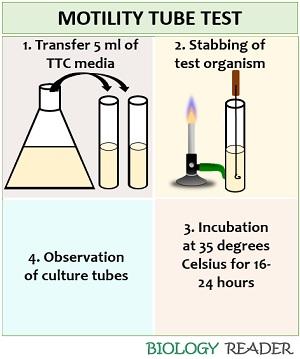
- Media preparation: Take all the ingredients required and weigh them accurately. Dissolve the contents in a sterile flask by adding the desired volume of sterile water. Autoclave the solution for 15-20 minutes at 121 degrees Celsius. After that, pour 5ml of the TTC media into each test tubes, and allow it to solidify.
- Bacterial inoculation: Take a sterile inoculating loop, and take out a well-isolated colony of the test organism straight into the media.
- Incubation: Allow the culture tubes to incubate at 35-37 degrees Celsius for at least 24-48 hours.
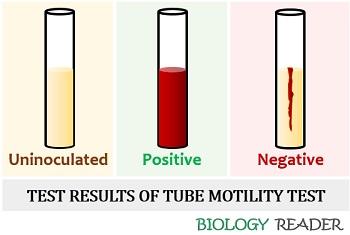
- Observation: Observe the tube for the diffusion of a red coloured zone around the line of inoculation.
- Test results: Diffused growth occurs around the stab line, which indicates a positive outcome. No growth around the route of inoculation represents a negative test result.
- Accuracy: The accuracy of the tube motility test depends upon the insertion of the inoculating needle (should be straight) and handling of the process.
Slide Test
It includes two methods, namely hanging drop and wet mount slide test.
Hanging Drop Method
It is a method of microscopic examination that confirms the motility of the organism. Hanging drop is a direct method that includes the following steps:
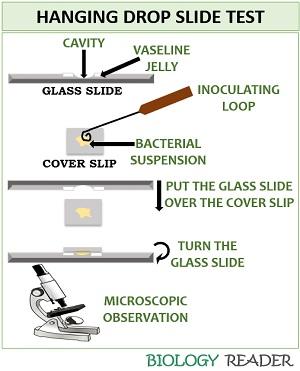
- Firstly, take a clean microscopic depression slide.
- Then, spread the vaseline petroleum jelly towards the vicinity of the depression wall.
- After that, take a clean, dust-free coverslip, and add a loopful of bacterial suspension towards the centre of the coverslip.
- After that, place the cavity region of the glass slide facing towards the bacterial suspension on the coverslip.
- Invert the glass slide and press the coverslip and cavity slide simultaneously.
- Finally, observe the glass slide under the microscope to check the bacterial flagella.
Wet Mount Method
It is also a direct method to identify the motility in bacteria. The wet mount method involves the following steps:
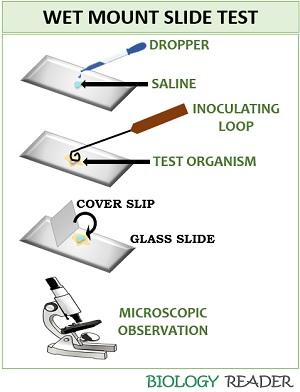
- Take a clean, dust-free microscopic glass slide.
- Add a drop of saline to the middle of the slide.
- Inoculate a well-isolated colony of the test organism and mix the inoculum with a drop of saline.
- Place coverslip to the one end of a glass slide, and slowly keep it over the test sample with the help of a needle.
- Press the coverslip to remove excess moisture or water bubbles by using blotting paper.
- At last, observe the glass slide under the microscope to check the bacterial motility.
Uses
The motility test discriminates the microorganisms as motile or non-motile, based on their motility. It also helps in the taxonomic classification of bacteria by focussing on the macroscopic and microscopic characteristics.
The motility test aids in the characterization or identification of pathogens or non-pathogens (as motility is one of the virulence factors) and facilitates species-level differentiation.
Example: Distinguishes between Enterococcus faecalis (non-motile) and Enterococcus gallinarum (motile).
Limitations
In the tube motility test, the inoculating needle should be taken out along the same line of inoculation to avoid false-positive growth. The water condensation in the tube motility test may lead to false-positive results. Weakly motile organisms may give contrast result interpretation.