Production of citric acid is an industrial process that uses raw materials like substrates, citric acid growth-promoting microorganisms and enzymes etc. for the commercial production of citric acid. Generally, the commercial production of citric acid works out best by employing the fermentation method.
Globally, there is around 7, 36,000 tonnes/year production of citric acid. The citric acid is commercially produced because of its broad applicability in human consumption (increased by 4% each year) and its high demand in food, pharmaceuticals, and other industries like cosmetics, toiletries etc.
In 1826, the commercial production of citric acid was first achieved by the John and Edmund Sturage Company, UK. In this context, you will get to know the definition, history, production process and recovery of the citric acid along with the uses and factors affecting the production process.
Content: Production of Citric Acid
- Definition
- History
- Production Process of Citric Acid
- Recovery of Citric Acid
- Uses
- Factors Affecting the Production
Definition
Production of citric acid is the process of extracting or manufacturing citric acid via natural and synthetic methods. The natural process is a technique through which we can extract citric acid naturally from the citrus plants like lemon, orange etc. Conversely, synthetic processes is an alternative technique through which one can produce citric acid chemically via enzymatic synthesis and biologically through the fermentation by the microorganisms.
What is Citric Acid?
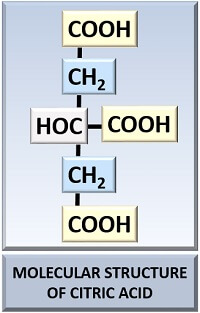
Citric acid is the common weak organic acid derived from the Latin word “Citrus”. The genus (Citrus) includes all the species of Lemon, orange etc. which are the best examples of having citric acid as the principal constituent.
Properties:
Let us look into the properties of citric acid in the table below.
| Properties | Citric Acid |
|---|---|
| Molecular formula | C6H8O7 |
| Molecular name | 2-hydroxy-1,2,3 propane tricarboxylic acid |
| Molecular weight | 210.14 g/mol |
| Occurrence | As a common metabolite in certain plants and animals |
| Melting point | 153 degrees Celsius |
| Solubility | Soluble in polar solvent like water, when citric acid is in pure form |
| Chemical state | Solid state at room temperature |
| Production | Either by chemical process or by microbial process (Fermentation) |
History
- 1874: Karls Scheels was the first scientist who isolated the citric acid from the lemon juice by crystallization.
- 1880: Grimoux and Adams were the two scientists who introduced the synthesis of citric acid from the glycerol.
- 1893: Wehmer carried out fermentation by taking Penicillium gaucum, where they observed the accumulation of citric acid as the by-product of calcium oxalate.
- 1917: Currie reported that the Aspergillus niger could yield citric acid at a higher rate in the sugar-based medium.
- 1922: Millard observed the accumulation of citric acid in the Aspergillus culture by limiting the nutrients in the bioreactor.
- 1965: For the citric acid production, yeasts were used along with the carbohydrates and alkanes as substrates.
- 1984: Abould Zeid and Ashy introduced the “Submerged fermentation technique” in the U.S. for citric acid production.
Production Process of Citric Acid
Production of citric acid can be accomplished by two methods like:
- A biochemical method by fermentation
- A biological method by chemical reactions
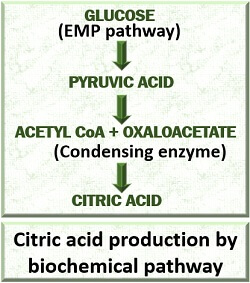
Biochemical Method
In a biochemical method, citric acid is produced as a primary metabolite by the microorganisms. The microorganisms produce citric acid during trophophase cell growth because of defective citric acid or Kreb’s cycle.
In defective Kreb’s cycle, a high amount of sugar is transported through the BMP pathway that forms acetyl CoA. The acetyl CoA condenses with oxaloacetic acid to yield “Citric acid” by the activity of an enzyme “Citrate synthetase”.
Therefore, in citric acid production, the enzymes of the Kreb’s cycle must be deactivated like an enzyme Aconitase/ Isocitrate dehydrogenase (causes further break down of the citric acid).
Biological Method
It involves the fermentation of citric acid by the use of microorganisms. The following three methods can carry out biological fermentation of citric acid:
Koji Process
It is called “Solid-state fermentation”. The koji process was first introduced in Japan. It is related to the use of agro-industrial residues for citric acid production. In Koji process, raw materials like apple pomace, sugar cane, beet molasses etc. are generally used. The Aspergillus niger utilizes raw materials.
The raw material’s pH and moisture content is adjusted to 4-5 and 70%, respectively. Then, cool the raw material under 30-60 degrees Celsius. After that, inoculate A. niger. After inoculation, the medium is transferred into large trays of 3-5 cm depth and incubated at 25-30 degrees Celsius for 3-7 days.
At last, the citric acid is extracted from the fermentation tank. The raw material’s starch content is degraded into citric acid by the amylase enzyme of the Aspergillus niger. The koji process does not require the substrate pre-treatment because the trace elements do not affect citric acid production.
Surface Culture Process
It is also called “liquid surface fermentation”. Surface culture fermentation was the first method introduced for citric acid production in 1919. In liquid surface fermentation, the culture medium (5-6 pH) is added to the aluminium shallow trays up to 5-20 cm deep.
The process is carried out in the fermentation chamber, which provides uniform air circulation, maintains relative temperature and humidity. First, the spores of A.niger is blown onto the surface of the culture medium for about 5-6 days, and dry air is then passed. Then, the pH of the culture medium is adjusted between 1.5-2 pH.
After 24 hours, the spores start to germinate, and the growth of white mycelium can be seen on the culture medium’s surface. After the utilization of sugar content by the moulds, the remaining liquid is separated from the mycelial mat. In the surface culture process, a small amount of citric acid is produced as a primary metabolite by the A. niger.
Submerged Culture Process
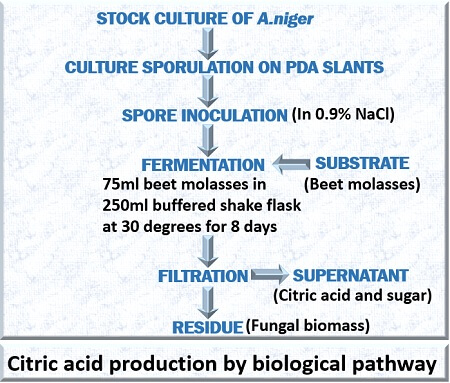
It is also called “Submerged culture fermentation”. About 80% of the citric acid production is carried out through submerged fermentation method. Submerged fermentation makes the use of black Aspergillus, i.e. A. japonicus. It is performed in a bioreactor made of stainless steel compiled with proper aeration, cooling system, impellers etc.
For carbon source, substrates such as beet molasses, corn starch etc. are used. For the nitrogen source, ammonia is used. The substrate used in this method requires pre-treatment like the addition of nutrients, sterilization etc.
The A. japonicus is inoculated into the culture medium and maintained at 30 degrees Celsius. Submerged fermentation is mostly carried out in a batch bioreactor in which 1500kg of citric acid and 500 kg of biomass can be produced from the 2500 kg glucose and 860 kg of oxygen.
Recovery of Citric Acid
The product formed after fermentation is the fermented liquor that looks hazy due to antifoaming agents, mycelia etc. Therefore to separate these things, a slurry of calcium hydroxide, i.e. Ca (OH)2 to form a precipitate of calcium citrate.
The precipitate of calcium citrate is filtered and washed. After filtration, treat a filtrate with the sulphuric acid for calcium precipitation as “Calcium sulphate” (CaSO4). Calcium sulphate is then treated with the activated carbon, by which it gets demineralized after passing it consecutively from the ion exchange bed.
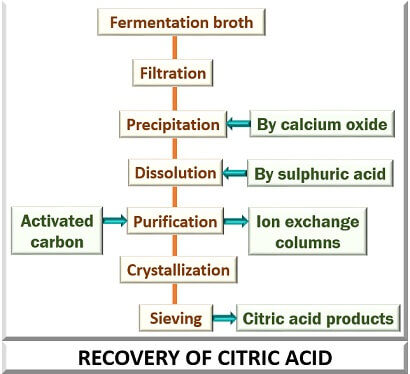
The solution obtained from this is subjected to the circulating crystallizers. The centrifugation then removes the crystals formed as a result of crystallization. After the completion of these steps, the remaining solvent is dried, sieved and then packed. The remaining mother liquor is again recovered through the same process.
Uses
The production of citric acid is necessary due to its wide applicability in the different fields like:
- Food industries: Citric acid is used to produce jams, jellies, candies, frozen fruits etc. In certain foods, citric acid is used as an artificial flavouring agent.
- Beverage industries: Citric acid is used for the production of soft-drinks and distilled beverages like wine.
- Hospitals: Citric acid is used as an effervescent agent at the time of blood transfusion.
- Cosmetic industries: Citric acid is used in cosmetic products like astringent lotions, hair gels etc.
Factors Affecting the Citric-Acid Production
Some factors can directly or indirectly affect the production process or the fermentation of citric acid.
Carbon Source Concentration
For most industrial production, carbon sources like glucose and sucrose are recommended, as they provide a good source of carbon for the growth of the biomass. Galactose acts as an alternative source of glucose and sucrose, but it allows low microbial growth and thereby does not favour citric acid cumulation.
Other carbon sources like cellulose, starch etc. allow limited growth and may slow down the microorganisms’ growth rate, which will lead to minimal production. The optimal sugar concentration ranges between 10-14%, and the sugar concentration below 2.5% will not produce citric acid.
Nitrogen Source Concentration
Ammonium salts like urea, ammonium sulphate etc. result in a decreased pH, crucial for the citric acid production. The nitrogen concentration must be in between 0.1-0.4 N/L. High nitrogen source will increase the microbial growth, which will consume more sugar and decrease the yield of citric acid.
Phosphorous Source Concentration
For the best production and growth of fungi, potassium dihydrogen phosphate is considered best for the phosphorous source. The concentration of phosphorous must be in between 0.5-5.0 g/l for the maximum yield. If phosphorous present in excess, it leads to the production of sugar acids that will decrease the carbon dioxide fixation and stimulate fungal growth.
Presence of Trace Elements
Divalent metals such as iron, zinc, manganese, copper etc. produce at the time of fermentation. If KH2PO4 is added to the zinc, it will favour the production, but the trace elements like manganese, iron, and high concentration of zinc leads to a reduction in yield.
Lower Alcohols Concentration
Lower alcohols like ethanol, methanol etc. enhance the citric acid fermentation. The concentration of lower alcohol must be in between 1-3%. Ethanol activates an enzyme Citrate synthetase activity, which doubles the time by decreasing 75% of Aconitase activity. Coconut oil (about 3%) also influences the citric acid production. Lower alcohols not only stimulate the citric acid production but also promotes the sporulation of microorganisms.
Miscellaneous Compounds
Calcium fluoride, sodium fluoride etc. are the miscellaneous compounds that accelerate the citric acid production, whereas potassium ferrocyanide decreases the yield.
pH Concentration
The pH of the culture medium is directly related to the growth of microorganisms and their metabolic activities. A pH changes to acidic by the microbial activity, like for Aspergillus, the pH drops to 3.0. The pH below 2.0 is optimal for the production of citric acid, and a pH of 2.2 is best for the growth of mould.
Aeration System
Aeration increases the dissolved oxygen concentration in the culture medium and thereby increases the yield of citric acid. The aeration system also reduces the fermentation time. The concentration of oxygen must be above 25% saturation. At the starting of the process, it must be 0.1-0.4 vvm otherwise, and it can produce excessive froth in the culture medium.