Protein denaturation is the deformation of a protein’s shape through some external stress. The external stress can be due to extremes of physical (heat, radiation etc.) or chemical agents (acids, alkalies etc.).
Denaturation in proteins is simply the unfolding of proteins due to the breakage of the bonds holding them. It significantly distorts the protein’s structural conformation. However, the amino acid sequence in proteins will not change. So, denaturation does not hamper the protein content or the nutritional values.
Based on structural complexity, proteins exist in primary, secondary, tertiary and quaternary structures. Except for primary proteins, all the proteins can be denatured via extremes of physical or chemical agents.
Proteins in their native states are the most functional and biologically active. Any physical or chemical change in native proteins leads them to lose their shape and biological activity and are called denatured proteins.
This post explains what protein denaturation is and examples of thermal denaturation. You will also know how the protein denatures by heat and what changes it brings to the protein.
Content: Protein Denaturation by Heat
- Definition of Protein Denaturation
- Examples of Denaturation
- Thermal Denaturation
- How Heat Denatures Proteins?
- Conclusion
Definition of Protein Denaturation
Do you know what proteins are? They form by the polymerization of different amino acids. To explain protein, we can take a reference of protein as a garland, in which the amino acids act like beads.
To understand “Denaturation”, let us break the term into ‘De’ and ‘Naturation’. The former means removal, and the latter means natural. So, we can define protein denaturation as the process in which the protein loses its natural or original characteristics.
Technically, protein denaturation is a biochemical process that refers to any structural deformation of the native protein by physical or chemical changes. A native protein has a complex, rigid structure and is functionally active.
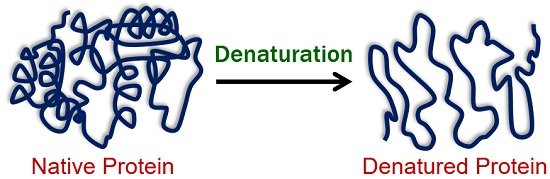
Denaturation can be seen only in the proteins’ quaternary, tertiary and secondary structures. It results in the loss of biological activity, making the protein functionally inactive.
Key Points
- Protein denaturants are the agents causing protein denaturation.
- Denatured protein is the protein that deforms due to the action of denaturing agents.
- Native proteins undergo physical, biological and chemical changes after denaturation.
- Renaturation is a process that refers to the regeneration of the denatured protein into its native form.
Examples of Denaturation
As the post is about protein denaturation by heat, let’s discuss some examples.
- The boiling of eggs denatures albumin proteins.
- Cooking meat denatures various proteins in the meat.
- Autoclaving glassware and media disrupt microbial proteins.
- Applying heated hair drying and styling devices denatures the hair’s keratin proteins.
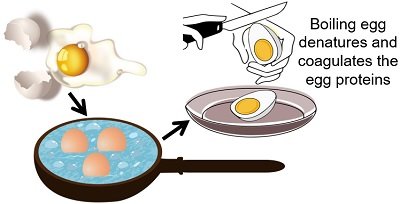
Boiling Eggs
On boiling, the physical state of eggs changes due to the coagulation of egg proteins. Most of the egg proteins in the egg white or albumin denature at a temperature of 60°C or above. On boiling, the albumin turns into a white, opaque and jelly-like substance. Thus, changes in viscosity and colour reflect the denaturation of egg albumin proteins.
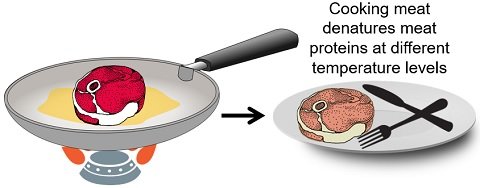
Cooking Meat
Cooking is another factor contributing to the denaturation of various protein-rich foods. For instance, cooking meat denatures protein strands, ultimately rendering meat moist and tender.
The proteins within the meat denature at various temperatures. Meat proteins start denaturing at roughly 105°F or above. You can see the change in the meat’s colour and texture as it passes through the various temperature stages.
- At 105°F temperature, the calpains begin to denature and lose activity.
- Above 125°F, you can observe a white opacity in meat due to the denaturation of heat-sensitive myosin.
- At 130°F, the light and red meat become pink.
- Further cooking (at 140°F) denatures the red myoglobin, forming a tan-coloured hemichrome. At this point, the colour and texture of the meat change noticeably. The pink colour of the meat turns brown and begins to shrink.
- At 160°F, connective tissues in meat melt away.
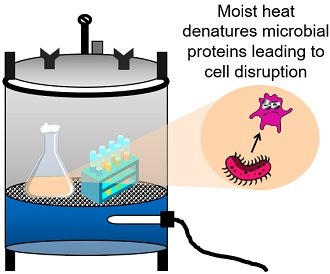
Autoclaving Equipment and Media
Autoclaving or steam sterilization is a popular method of killing microbes and their spores. At 120°C temperatures, the steam generates moist heat. This heat is sufficient to denature the microbial proteins, making them functionally inactive.
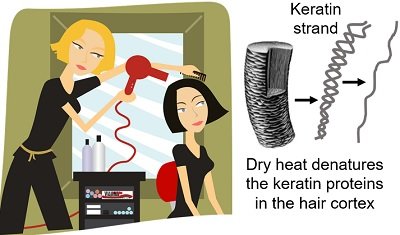
Drying and Styling of Hairs
Keratin is the primary protein found in the hair cortex. We know that the frequent usage of hair dryers and hair styling devices damages the hair.
Temperature above 140°C alters the shape of keratin strands from α-keratin to β-keratin. Due to this, hairs lose elasticity, and the hair shaft weakens, leaving it prone to damage.
Thermal Denaturation of Proteins
Different alpha amino acids function as bricks to build the protein. So, protein is a polymer or polypeptide of more than 50 amino acids.
- The primary structure of a protein has a linear structure with a unique sequence of amino acids.
- The secondary structure of protein exists in two conformations, alpha-helix and beta-sheets. The polypeptide chain is twisted.
- Protein’s tertiary structure has a 3D conformation and a more folded appearance.
- The quaternary structure of protein comprises two or more subunits of polypeptide chains.
Several forces are responsible for holding up the native structure of the protein. Hydrogen bonds, ionic interactions, disulfide bridges, and hydrophobic interactions are the covalent and non-covalent forces stabilizing the protein structure.
Thus, heat that alters protein conformation by disrupting these forces is called thermal denaturation.
How Heat Denatures Proteins?
Heat exposure above 50°C generally denatures the proteins. Heat increases kinetic energy, causing atoms of proteins to vibrate. The vibrating motion disrupts or breaks the weak hydrogen bonds and dispersion forces.
Eventually, the rigid, folded, stable conformation of protein will unfold. Denaturation is associated with alterations in properties like physical, biological and chemical properties. Following are the changes resulting from thermal denaturation:
- Loss of protein’s helical structure.
- Loss of protein’s biological activity.
- Changes the solubility of the native protein.
- Causes precipitation or coagulation of proteins.
Depending upon these changes, denaturation can be irreversible or sometimes reversible.
- When the denatured proteins can not get back to their original shape called irreversible denaturation. Example: Making an omelette from an egg.
- If the denatured proteins regain their native form, called reversible denaturation. Example: Temporary hair straightening and curling of hairs.
Renaturation is the reversal mechanism of denaturation. It allows the reconstruction of disrupted proteins to their original state.
Conclusion
Protein denaturation by heat or other physical or chemical extremes can break the covalent and non-covalent bonds holding the polypeptide chains. It eventually unfolds the protein, or the polypeptide chains become disordered, making the protein non-functional.
But, the nutritional values remain intact, or the protein content will not change. So, protein denaturation is significantly important for us, as the enzymes in our digestive system find the denatured proteins easy to digest.