Capsule staining is one of the staining methods, which creates contrast in the microscopic image either by staining bacterial cells and background or staining the capsule only. Therefore, capsule staining can be done by two methods, namely positive and negative staining methods. Positive staining method either stain a capsule only or stain both the capsule and bacterial cell.
The negative staining method stains both the bacterial cell and its background. The capsule staining is employed to know the presence or absence of the capsule, after which you could classify the type of bacteria, i.e. whether a bacteria is capsulated or non-capsulated.
It is necessary to perform capsule staining because a capsule’s presence indicates a virulent strain of bacteria that can cause disease. Thus, by knowing the presence of the capsule, we can determine the degree of pathogenicity. Here, you will get to know the definition, principle, different methods and significance of the capsule staining, along with the meaning of bacterial capsule.
Content: Capsule Staining
Definition of Capsule Staining
A capsule staining is a special staining method, which uses differential capsule stains that either highlight the capsule or stains the bacterial cell along with the background. It is an important staining method because some bacteria like Bacillus anthracis, Streptococcus pneumoniae etc., have a capsule, which can cause pathogenicity in humans and animals. Thus, it becomes necessary to identify the presence of an extracellular capsule. Capsule staining can be performed by employing India ink, Anthony’s, Maneval’s and Hiss method.
Bacterial Capsule
A bacterial capsule refers to the mucilaginous coating that surrounds the cell wall of bacteria. The capsule or glycocalyx is composed of glycoproteins. The cytoplasm of bacteria partially forms a capsule, which then migrates to the cell wall, and there it exists as a mucous or slime covering.
A bacterial capsule protects a cell from desiccation because of its mucous content. A mucus layer also protects a cell from phagocytosis. The capsule also acts as a virulence factor and is responsible for the pathogenicity of many microorganisms like Streptococcus pneumoniae, Escherichia coli, Neisseria meningitis etc.
In the majority, a capsule consists of polysaccharides, but a few may possess polypeptides or glycoproteins. The formation of a capsule is a process controlled genetically. Capsules are the structures clearly visible under the light microscope after they get stained via differential capsule stain.
Bacteria may possess a capsule (capsulated bacteria) or lack a capsule (non-capsulated bacteria). Capsule are of two types, namely micro and macro capsule. The microcapsule has a size of less than 0.2 µ, whereas the macrocapsule has a size of more than 0.2µ.
The bacterial capsule is non-ionic in nature, i.e. it will neither take up the colour of acidic stains nor basic stains. The basic dye will stain the negative bacterial cell, and an acidic stain will stain the positive background. To stain a capsule, we need a special capsule stain that can focus on the capsule.
A capsule can be easily destroyed by heat treatment, due to which a step of heat fixing is skipped in capsule staining. In addition to this, avoid steps like washing or rinsing because it can dislodge the bacterial cell capsule. To enhance the capsule’s size or increase its visibility, we can also add a drop of serum. The addition of serum provides a more unobstructed view of a capsule under a light microscope.
Principle
The principle of capsule staining is based on staining of background with an acidic stain and staining of bacterial cell with a basic stain. A capsule being non-ionic will not stain by either of the two dyes. Thus, capsule staining creates contrast by staining a bacterial cell along with its background and leaving a capsule as a colourless halo. The other approach of capsule staining is to stain the capsule by leaving a bacterial cell and background colourless. Differential capsule stains are available to highlight the specific structure (like capsule) in a bacterial cell.
Methods of Capsule Staining
The capsule staining employs various techniques, among which the most common methods are:
- India ink method
- Anthony’s method
- Maneval’s method
- Hiss method
India ink Method
India ink method uses two types of stain, i.e. a basic stain (Crystal violet) and an acidic stain (India ink). Crystal violet is a positive stain that will stain the negatively charged bacterial cell. India ink is a negative stain that will stain the positively charged background. After staining:
- The background seems darker or black.
- A bacterial cell appears violet.
- The capsule seems like a clear halo.
Procedure
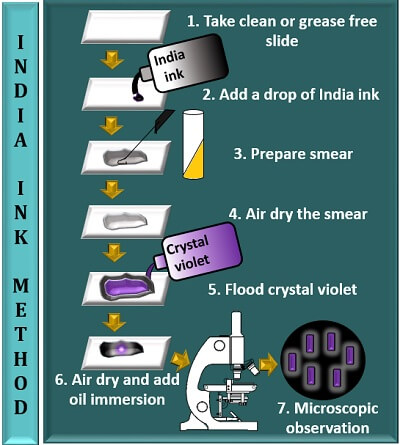
India ink method involves the following steps:
- Take a clean, sterilized or grease-free slide.
- Add a drop of India ink to the centre of the glass slide.
- Prepare a smear by taking an inoculum from the bacterial culture and mix it with a drop of India ink.
- Then, allow the smear to air dry (do not heat fix the smear, as it may result in cell shrinkage that in turn can distort the bacterial capsule).
- Flood a smear with the crystal violet stain and leave it for 30 seconds. Later, remove the extra stain by tilting a glass slide.
- Add oil immersion to the stained area and observe it under the microscope using a 100X objective.
India ink method is a type of negative staining method, which stains both the bacterial cell and its background (but not a capsule). As a result, a capsule appears as a bright halo between the violet bacterial cell and a darker background.
Anthony’s Method
This method makes the use of two reagents, namely crystal violet as a primary stain, 20% of CuSO4 solution as a decolouring agent and counterstain. Crystal violet stains the bacterial cell and background. The CuSO4 solution stains the non-ionic capsule. After staining:
- A bacterial cell appears violet.
- The background appears light violet.
- The capsule appears as a faint blue halo.
Procedure
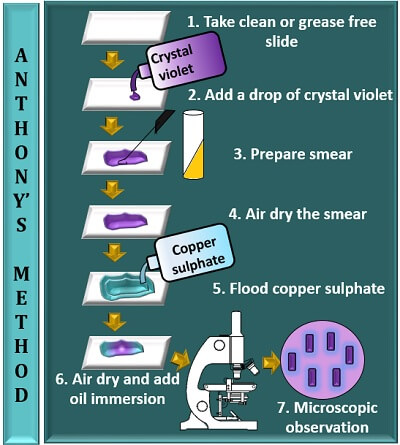
Anthony’s method involves the following steps:
- Take a clean, sterilized or grease-free slide.
- Add a drop of crystal violet to the centre of the glass slide.
- Prepare a smear by taking an inoculum from the bacterial culture and mix it with a drop of crystal violet.
- Then, allow the smear to air dry (do not heat fix the smear).
- Flood a smear with 20% of CuSO4 solution and leave it for at least 30 seconds. Later, remove the extra stain by tilting a glass slide.
- Add oil immersion to the stained area and observe it under the microscope using a 100X objective.
Anthony’s method is a type of positive staining method, which stains the capsule along with the bacterial cell (not the background). As a result, a capsule appears as a faint blue halo between the violet bacterial cell and purple background.
Maneval’s Method
This method makes the use of two reagents, i.e. acidic stain (Congo red) and a special stain (Maneval’s stain). The composition of Maneval’s dye includes:
- 10% Ferric chloride (acts as a mordant).
- 5% phenol (increases the penetration of the stain between the smear).
- Acid fuschin (stains the bacterial cell being a basic dye).
- Acetic acid (decreases the pH of the smear to the acidic side).
- Congo red appears red (at neutral pH) and appears blue in colour (at acidic pH).
After staining, we could see the specimen with the following microscopic observation:
- A bacterial cell appears bright red-pink.
- The background appears dark blue in colour.
- The capsule appears as a clear halo.
Procedure
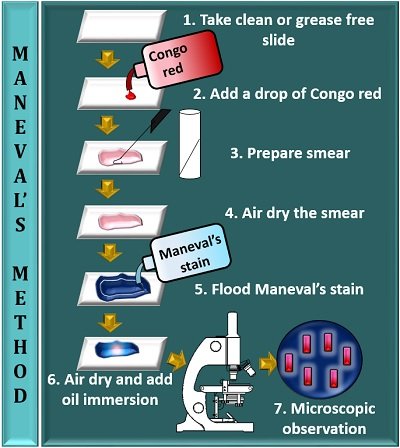
Maneval’s method involves the following steps:
- Take a clean, sterilized or grease-free slide.
- Add a drop of 1% Congo red to the centre of the glass slide.
- Prepare a smear by taking an inoculum from the bacterial culture and mix it with a drop of Congo red.
- Then, allow the smear to air dry (do not heat fix the smear).
- Flood a smear with Maneval’s stain and leave it for at least 1 minute. Later, remove the excess stain by tilting a glass slide.
- Add oil immersion to the stained area and observe it under the microscope, having a 100X objective.
Maneval’s method is also a type of negative staining method, which stains the bacterial cell and its background, but not a capsule. As a result, a capsule appears as a clear halo between the pink bacterial cell and blue background.
Hiss Method
This method uses two reagents, namely crystal violet and copper sulphate solution. After staining:
- A Bacterial cell appears dark violet.
- The background seems brighter in colour.
- The capsule appears as a light violet colour.
Procedure
Hiss method involves the following steps:
- Take a clean, sterilized or grease-free slide.
- Add a drop of crystal violet to the centre of the glass slide.
- Prepare a smear by taking an inoculum from the bacterial culture and mix it with a drop of crystal violet.
- Then, allow the smear to air dry (do not heat fix the smear).
- Flood a smear with copper sulphate solution and remove the excess stain by tilting a glass slide.
- Add oil immersion to the stained area and observe it under the microscope, having a 100X objective.
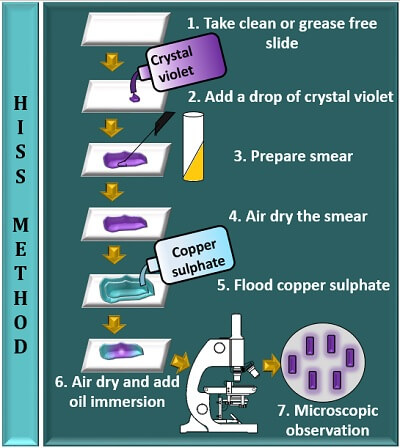
The Hiss method is also a type of positive staining method that stains the capsule and the bacterial cell with a brighter background. As a result, a capsule appears as a light violet colour between a dark violet coloured bacterial cell and colourless background.
Significance
Capsule staining allows us to classify bacteria based on the presence or absence of a capsule. A bacterial capsule acts as a virulence factor and gives pathogenicity to the bacterial cells.