The difference between hypertonic and hypotonic solution is mainly due to the factors like:
- Solute concentration
- Solvent concentration
- Effect on a cell
A hypertonic solution has a high solute concentration, whereas hypotonic solution has a low solute concentration. The concentration of the solvent is low in the hypertonic solution and high in the hypotonic solution. When a cell is kept inside a hypertonic solution, it shrinks. Oppositely, a cell becomes turgid when kept in the hypotonic solution.
Here, we will discuss the key differences between the hypertonic and hypotonic solution along with the comparison chart. Besides, you will also get to know the definition, the difference in the cell tonicity and the effects of the hypertonic and hypotonic solution to the cell.
Content: Hypertonic Vs Hypotonic Solution
Comparison Chart
| Properties | Hypertonic solution | Hypotonic solution |
|---|---|---|
| Meaning | In this, solution outside the cell has higher solute concentration than the fluids inside the cell | In this, solution outside the cell has low solute concentration than the fluids inside the cell |
| Osmotic pressure | High | Low |
| Solute concentration | High | Low |
| Solvent concentration | Low | High |
| Effect on the cell | It causes cell shrinkage | It causes cell swelling |
| Type of osmosis | Exosmosis occurs as the water exits the cell | Endosmosis occurs as the water enters the cell |
| Application | Haemorrhage, burn, cerebral edema etc. | Dehydration, hypernatremia etc. |
| Intravenous solution | D5 NaCl, D5 LR, D5 0.45% NaCl etc. | D5W, 0.25% NaCl, 2.5% dextrose etc. |
| Usefulness in food preservation | It is helpful | It is not helpful |
Definition of Hypertonic Solution
Hyper means high concentration, and tonic means fluid. It is defined as the solution with a high solute concentration outside than the fluids inside the cell, and a high water concentration inside the cell than the surrounding fluid. Hypertonic solution results in cell crenation or contraction.
Definition of Hypotonic Solution
Hypo means low concentration, and tonic means fluid. It is defined as the solution with a low solute concentration outside than the fluids inside the cell, and a high water concentration outside the cell. Hypotonic solution results in puffing up or expansion of the cell.
Difference in Tonicity
Tonicity can be defined as the phenomena that govern water flow through a semipermeable membrane through the relative concentration of solutes mixed in the solution. To know the difference in the tonicity of the hypertonic and hypotonic solution, let’s take an example of three different solutions with different solute-solvent concentration.
The solute will be the compound dissolved in a solvent and solvent will be the surrounding fluid. By knowing the solute concentration and solvent concentration, we can determine the solution, whether it is hypertonic or hypotonic.
The solute concentration of the first solution is 30% with a solvent concentration of 60% NaCl. The solute concentration for the second solution is 80% with a solvent concentration of 60% NaCl, and the third solution is having the same solute-solvent concentration of 60% NaCl.
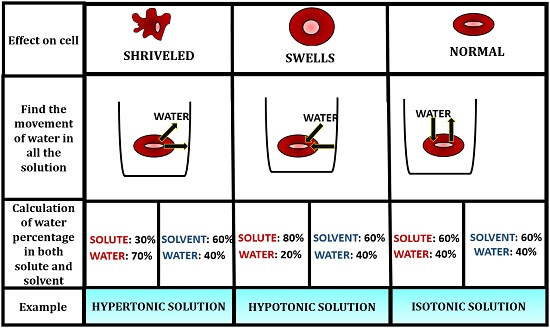
Calculation of water percentage
Now, to know whether the solution is hypertonic or hypotonic, we have to calculate the water percentage in both solute and solvent by subtracting it with 100.
We will have our readings, where the water concentration in a solute will be 70%, and the concentration of water in a solvent will be 40%, in the first solution. Then, the water concentration in a solute will be 20%, and the concentration of water in a solvent will be 40%, in the second solution. The water concentration in a solute will be 40%, and the concentration of water in a solvent will be 40%, in the third solution.
Determination of Water Movement
After this, we must find the flow of water molecules in all these three solutions. To know the movement of water, we must know the concept of osmosis. Osmosis is the process of water movement from the region of their high concentration to the region of their low concentration through a semipermeable membrane.
- Hypertonic solution: The water will move from the higher concentration of water in a solute to the lower concentration of water in the solvent through a cell’s semipermeable membrane, to maintain the equilibrium.
- Hypotonic solution: From the second solution, water will move from the higher concentration of water in a solvent to the lower water concentration in the solute through a semipermeable membrane of the cell, to maintain the equilibrium.
- Isotonic solution: In the third solution, the concentration of solute and solvent will be the same. Thus, the water will move inside and outside the cell to the same extent through a semi-permeable membrane to maintain the equilibrium.
Effect on the Cell
The hypertonic and hypotonic solution affect the cell by changing its structural configuration.
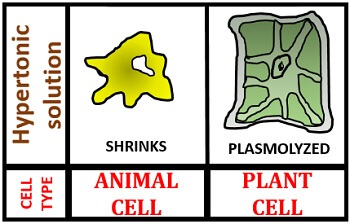
In a hypertonic solution, the cell shrinks because of the high concentration of water inside the cell. Therefore, water will move out from the cell into its surroundings to maintain the equilibrium both outside and inside the cell. This type of water movement is termed as exosmosis, which causes cell crenation. The animal cell becomes shrivelled, and a plant cell becomes plasmolyzed as we could see in the image given below.
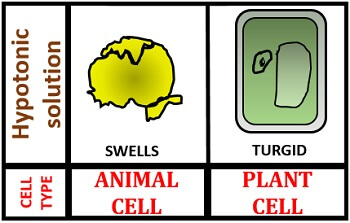
In a hypotonic solution, the cell swells because of the high concentration of water outside the cell. Therefore, water will move into the cell from its surroundings to maintain the equilibrium both outside and inside the cell. This type of water movement is termed as endosmosis. In a hypotonic solution, the animal cell becomes swelled, and a plant cell becomes turgid as we can observe in the picture given below.
Key Differences Between Hypertonic and Hypotonic Solution
- A hypertonic solution has high osmotic pressure, whereas a hypotonic solution has low osmotic pressure.
- The concentration of solute is more in hypertonic solution than the hypotonic fluid.
- The concentration of solvent is low in hypertonic and high in hypotonic.
- A cell becomes shrivelled in a hypertonic solution, while A cell gets swelled in a hypotonic solution.
- The movement of water in the hypertonic solution is from a cell to the surrounding, i.e. exosmosis, and the movement of water from the surrounding into the cell, i.e. endosmosis occurs in a hypotonic type.
Conclusion
Both the hypertonic and hypotonic solutions are based on the concept of tonicity and osmosis. By knowing the concept of tonicity and osmosis, we can understand the general idea about the direction and extension of the water movement in the solution.
Excellent blogger, Thank you for publishing this great post. I found it handy. Best regards !!
Very Cool, keep up the good work like this.