The pH scale is a measurement of the acidic or basic behaviour of the aqueous solution. It clearly denotes the compounds as acidic and basic, when these have pH between 0-6 and 8-14, respectively. A pH scale is an important tool for differentiating the acidic and alkaline products, depending on the colour change that varies according to the different pH values.
The intensity of the acidic products increase as the pH decreases from 6 to 0, i.e. lower is the pH value higher will be the acidity. In contrast, the intensity of the alkaline products increase as the pH increases from 8 to 14, i.e. higher is the pH value, lower will be the alkalinity. Neutral compounds are indicated by pH 7.
Content: pH Scale
Definition of the pH scale
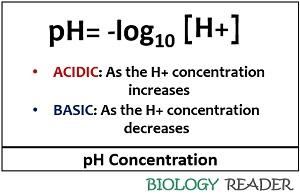
The pH scale can be defined as the logarithmic scale, which measures the acidity or alkalinity of the solution depending upon the hydrogen ion concentration. It was introduced by a scientist named SPL Sorenson in the year 1909. As the proton concentration increases, it will indicate the acidic behaviour of the given solution. As the proton concentration decreases, it will represent the basic behaviour of the given solution.
 On a pH scale, the acidity and alkalinity increase or decreases logarithmically. For instance, the acidic behaviour of solutions having pH 1 will be 10 times more than the aqueous solutions having the pH value 2 and 100 times more than the solution having the pH value 3. Similarly, the alkalinity of the solution having pH 14 will be 10 times more than the solution having a pH 13 and 100 times more than the solution having a pH of 12.
On a pH scale, the acidity and alkalinity increase or decreases logarithmically. For instance, the acidic behaviour of solutions having pH 1 will be 10 times more than the aqueous solutions having the pH value 2 and 100 times more than the solution having the pH value 3. Similarly, the alkalinity of the solution having pH 14 will be 10 times more than the solution having a pH 13 and 100 times more than the solution having a pH of 12.
- pH: The lowercase letter ‘p’ is an acronym for the term, i.e. the power or potential and the uppercase letter ‘H’ is an acronym for the hydrogen ion.
- Log10: The pH is a logarithmic scale in which any change in an integer value or change in each one unit will change the H+ concentration by the factor of 10.
- [H+]: Here, the square bracket designates the concentration, and the H+ ion denotes the proton or hydrogen ion.
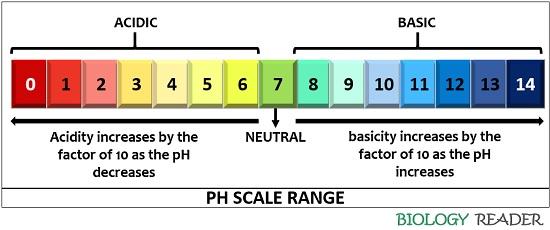
pH Range
The pH varies between the values ranging between 0-14, but the values may exceed for the extremely acidic and alkaline solution. Therefore, the pH scale has no upper and lower limit or the values may exceed above 14 and decrease below 0. A concentration of H+ and OH- ions decide either the given solution is acidic or basic. Higher will be the concentration of H+, more acidic will be the compound, and higher is the concentration of OH-, the solution will be more basic.
pH Scale Examples
The pH value ranges between 0-14, so different solutions will show varying pH depending on their physicochemical properties.
- pH -0: Solutions that are having pH -0 are extremely acidic, and it includes battery acid.
- pH -1: It is 10 times less acidic than the solution having pH-0, and it includes gastric acid.
- pH -2: It is 100 times less acidic than the solution having pH-0, and it includes lemon juice, vinegar etc.
- pH -3: It is 1000 times less acidic than the solution having pH-0, and it includes orange juice, soda etc.
- pH -4: It is 10000 times less acidic than the solution having pH-0, and it includes tomato juice, acid rain etc.
- pH -5: It is 100000 times less acidic than the solution having pH-0, and it includes black coffee, banana etc.
- pH -6: It is 1000000 times less acidic than the solution having pH-0, and it includes urine, milk etc.
- pH -7: Distilled water is a common example that is having a neutral pH. The human blood and the cytosol of the cells have a slightly neutral pH like 7.4 and 6.8, respectively.
- pH -8: It includes slightly basic seawater.
- pH -9: It is 10 times more basic than the solution having pH-8, and it includes baking soda.
- pH -10: It is 100 times less acidic than the solution having pH-8, and it includes milk of magnesia.
- pH -11: It is 1000 times less acidic than the solution having pH-8, and it includes an ammonia solution.
- pH -12: It is 10000 times less acidic than the solution having pH-8, and it includes a soap solution.
- pH -13: It is 100000 times less acidic than the solution having pH-8, and it includes bleach.
- pH -14: It is 1000000 times less acidic than the solution having pH-8, and it includes Lye.
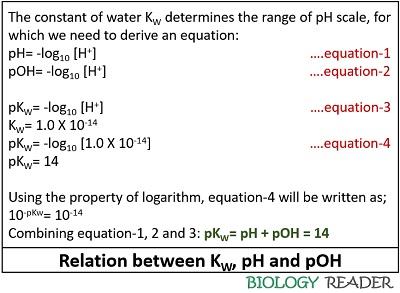
The relation Between KW, pH and pOH
To understand the acidity and alkalinity of any solution, water is the best example. Water being amphiprotic in nature, can behave as both an acid and a base. The water equilibrium constant Kw is related to pH and pOH and can be derived as the image given below:
Important Points
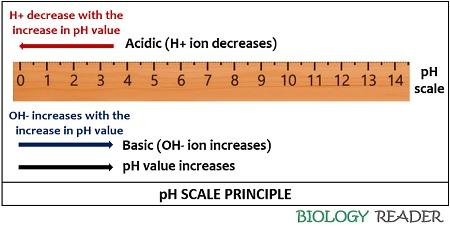
The pH Scale provides a range of acidic, neutral and basic solutions between 0-14. The pH scale is directly related to the OH- concentration while inversely related to the H+ concentration. A pH value increases as the OH- ion concentration increases, while the H+ ion concentration decreases with the increasing pH value.
By adding acid in the water, the water equilibrium switch towards the left, i.e. the OH- concentration decreases. Similarly, the water equilibrium shifts towards the right, i.e. the H+ concentration decreases on the addition of base in the water. The pH unit is temperature-dependent.
Autoionization of water is the property through which the acidic and basic behaviour can be easily understood based on proton acceptance and donation. Molecules that accept protons or H+ ions are said to be basic, while those giving protons are said to be acidic. Water is amphiprotic in nature, which behaves both as an acid and a base by forming hydronium and hydroxide ions on self-ionization.
To measure the pH of any solution, it must be aqueous or contain water. As the pH of oil, alcohol etc., cannot be measured. The pH scale is a universal measure, which can calculate the amount of free hydrogen and hydroxide ions in the given solution. The pH of the solution can be affected by the change in temperature, the addition of acids and bases etc.
Conclusion
Therefore, we can conclude that the pH scale is a measure of H+ and OH- concentration in the aqueous solution. Acidic solutions that yield H+ ions have lower pH values, whereas basic solutions that give off OH- ions have higher pH values. The study of pH is not only important in chemical science but also in many other fields like biochemistry, medical science etc.