The differences between the paper and thin layer chromatography are due to the following properties like:
- The principle for the particle separation
- Use of stationary phase
The main principle behind the particle separation in the paper chromatography is partition type, whereas in thin layer chromatography it is adsorption type.
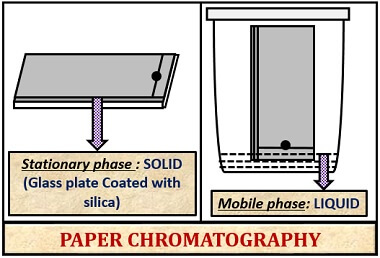
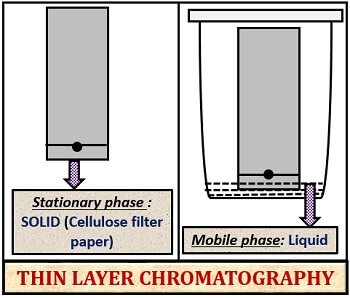
Paper chromatography makes the use of cellulose filter paper as the stationary phase. In contrast, thin layer chromatography makes the use of glass plate coated with silica gel as the stationary phase.
Content: Paper Vs Thin Layer Chromatography
Comparison Chart
| Properties | Paper chromatography | Thin layer chromatography |
|---|---|---|
| Principle | Partition chromatography | Absorption chromatography |
| Preparation time | Less | Comparatively more |
| Stationary phase | Water present in the pores of cellulose | Glass coated with silica gel |
| Mobile phase | Hydrophilic mobile phase: Isopropanol: Ammonia: Water Methanol: Water Hydrophobic mobile phase: Dimethyl ether: Cyclohexane Kerosene: Isopropanol | Pyridine, carbon-tetrachloride, acetone, glycerol etc. |
| Sample requirement | Less amount of sample is required | Comparatively requires little more sample |
| Heat treatment | Paper cannot be heated in an oven for long time | TLC plate can be heated in an oven for long time |
| Use of silica gel | It does not use silica gel | It makes the use of silica gel |
| Separation efficiency | More efficient for polar water soluble compounds. | Efficient for less polar compounds |
| Physical separation | Physical separation can be done, and it prefers ascending technique | It lacks the physical separation and it prefers descending technique |
| Use of corrosive reagents | Can’t use | It can be coated with corrosive reagents |
| Evaluation | Cannot evaluate under the UV-light | Spots can be evaluated under the UV-light |
| Time | It takes less time for the particle separation | It takes more time for the particle separation |
| Cost | Cheap | Comparatively costly |
What is Chromatography?
It is the analytical method of separating different mixtures of the component from the stationary and mobile phase. In the chromatography technique, the different mixtures of a component will appear as different spots on the adsorbent. The sample moves upward through the capillary action of the mobile phase.
This technique was first introduced in 1903 by the scientist M.S. Tswett. Therefore, chromatography is merely the separation technique that separates the different components of a molecule by making the use of two distinct phases, namely a stationary and mobile phase.
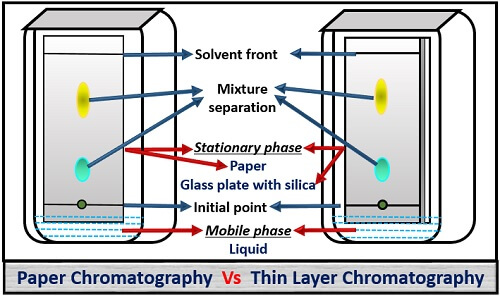
- Mobile phase: It is the carrier or matrix, in which the solvent runs upwards through the stationary phase.
- Stationary phase: It is the adsorbent that stays fixed inside the developing chamber.
Definition of Paper Chromatography
It is the type of Solid-liquid partition chromatography, in which the stationary phase is the cellulose filter paper, and the mobile phase is liquid, where the particles are separated based on their polarity towards both the phases.
Definition of Thin Layer Chromatography
It is the type of Solid-liquid adsorption chromatography, in which the stationary phase is the glass plate coated with the silica gel, and the mobile phase is liquid, where the particles are separated based on their polarity towards both the phases.
Process of Paper Chromatography
It involves the following steps:
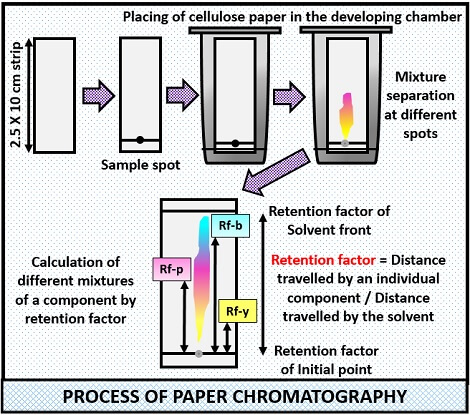
- First, take the sample containing the different mixture, for example (leaf extract).
- Then, take a piece of cellular filter paper of size having 2.5 cm breadth and 10 cm length.
- After that, make a line 1.5 cm above from either end of the filter paper, with the help of a pencil.
- Put a small drop of the sample at the centre of the line.
- Then, take a non-polar solvent (mobile phase) will move upwards through the capillary action.
- After that, hang the filter paper in the beaker in such a way that it slightly touches the surface of the solvent and finally cover the beaker with the glass lid for better results.
- Then, wait for a few minutes to see the separation of a mixture in the form of different spots on a paper.
- Take out the filter paper, let it dry and note down the readings to calculate the Rf value of each mixture in the sample.
Process of Thin Layer Chromatography
It involves the following steps:
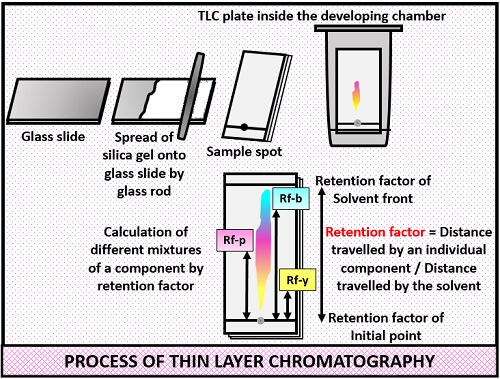
- Firstly, take a clean glass slide.
- Then, prepare the adsorbent by making a slurry of silica gel.
- Make the thin slurry of silica gel and spread it uniformly on the glass plate with the help of a glass rod.
- After that, give a heat treatment to the glass plates under an oven for 15-20minutes to solidify the silica gel.
- Then, put a small drop of sample, 1 cm above on either end of a glass plate.
- Place the glass plate in the developing chamber and cover the glass lid to prevent evaporation.
- After the solvent run the appropriate distance, note down the Rf values of each spot.
Key Differences Between Paper and Thin Layer Chromatography
- The principle behind the paper chromatography is based on Partition. The principle behind the thin layer chromatography is based on Adsorption.
- Paper chromatography requires less preparation, whereas thin layer chromatography requires more preparation time.
- The stationary phase of paper chromatography is the water trapped in the cellulose filter paper. The stationary phase of the thin layer chromatography is the glass plates coated with silica gel.
- Thin-layer chromatography makes the use of silica gel, whereas paper chromatography does not.
- Corrosive reagents can be used in thin layer chromatography, while the corrosive agents can destroy the paper.
- The different spots can be seen under the UV-lamp in the thin layer chromatography, but not in the case of paper chromatography.
- Paper chromatography requires less time for particle separation, whereas thin layer chromatography requires more time.
Similarities
- Both paper and thin layer chromatography are having a solid stationary phase.
- Both have the liquid mobile phase.
- The particle separation in both the types is based upon the polarity of the molecules to the stationary and mobile phase.
Conclusion
In this article, we have discussed the differences in properties, differences in the process and the key differences between the paper and thin-layer chromatography. The particles separate over the stationary matrix based on their adsorption to the stationary phase or solubility in the mobile phase.
There will be more attraction of the polar solute towards the polar adsorbent (cellulose and silica gel) and will not move upwards with the less polar solvent. The non-polar solute will attract more towards the less polar solvent, i.e. to the mobile phase and will move upwards by the capillary action.
The different spots will appear after the completion of the chromatograph, after which Rf factor is calculated. Rf factor is also called Retention or Retardation factor. Retention factor is equal to the distance travelled by the component divided by the distance travelled by the solvent.
Very good presentation for quick & Fast understanding about the techniques to refresh the subject
Thank you, it is easy to understand.
I love this content.
Thanks for providing such quality content.
Thank you for making such a great post! I’ve been having problems understanding this subject for a long time. After I read your article, it became crystal clear to me! Thank you so much again!