The electron transport system refers to the “Electron transport chain” or “ETS” (in abbreviated form) that is present in the inner mitochondrial membrane. ETS involves electron transfer through a series of protein complexes from higher (NADH+) to lower energy state (O2) by releasing protons into the cytosol.
A movement of proton or H+ from a matrix to cytosol generates a proton motive force and creates an electrochemical gradient. Then, the proton molecules tend to diffuse down the electrochemical gradient again into the mitochondrial matrix and releases ATP via ATP synthase.
An oxygen atom is the last carrier, which accepts the electron and combines with the free hydrogen ions in the mitochondrial matrix to give water. Thus, the oxygen carrier maintains the membrane potential by removing the de-energized electrons from the inner mitochondrial membrane.
In this lesson, we will discuss the definition, components, location and mechanism of electron transport system. You would also get to know the step by step explanation of the ETS.
Content: Electron Transport System
Definition of Electron Transport System
It refers to the mechanism of cellular respiration that occurs in the inner mitochondrial membrane. It is the third and last stage of cellular respiration. Electron transport chain or Respiratory chain is the alternative terms of the electron transport system. ETS follows an aerobic pathway. In ETS, the electrons flow from a high to low energy state and finally leaves the inner membrane space. The oxygen carrier combines with free protons to produce waste (water).
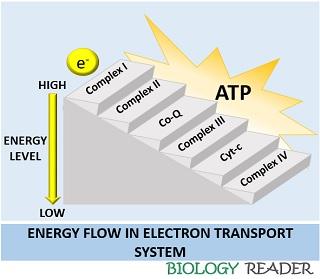
The electron transport system consists of hydrogen carrier complexes, electron carriers and an ATP synthase ion channel. ETS creates a chemiosmotic gradient, which allows the diffusion of protons into the matrix by the production of ATP. The ATP is harnessed by the cell to perform cellular and metabolic activities.
Step by Step Explanation of Electron Transport System
The electron transport system can be summarized into the following steps:
Step 1: Generation of proton motive force
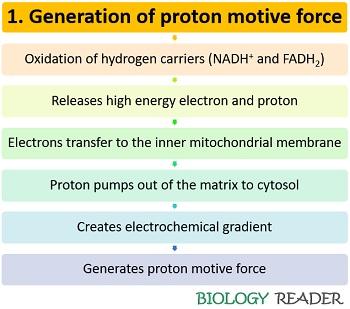
In the first step of the electron transport chain, the NADH+ and FADH2 molecule of glycolysis and Kreb’s cycle is oxidized into NAD+ and FAD, respectively, along with the release of high energy electrons and protons. The electrons diffuse into the inner mitochondrial membrane that consists of a series of large protein complexes.
The passage of electrons from one carrier protein to another results in the loss of some energy or ATP. The ATP is then used up by the protein complexes to move the protons from the matrix to the intermembrane space. Thus, the diffusion of protons across the inner mitochondrial membrane is mediated via chemiosmosis, which creates a proton motive force across the electrochemical gradient.
Step 2: Synthesis of high energy molecule ATP
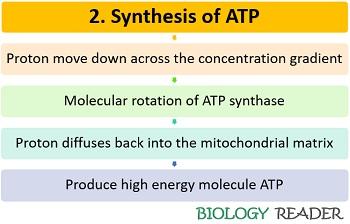
The H+ ions generate a proton motive force that facilitates the downhill movement across the concentration gradient of the inner mitochondrial membrane. H+ ions tend to diffuse back into the mitochondrial matrix through the channel proteins via a transmembrane enzyme (ATP synthase), and thereby producing ATP.
Step 3: Oxygen reduction
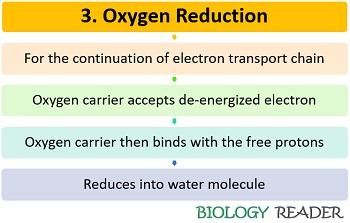
For the continuation of the electron transport system, the de-energized electrons must be release out via an electron acceptor O2 molecule. Oxygen accepts the electrons from the fourth complex. Eventually, the oxygen carrier associates with the free protons and reduces to yield H2O.
Components of ETS
The electron transport system is the combination of the following elements:
Complex I
It is composed of flavin mononucleotide and iron-sulphur protein. Complex I or “NADH dehydrogenase” oxidizes NADH+ into NAD+ and releases two electrons and four protons. NADH dehydrogenase pumps out four protons from the matrix to the cytosol and transfers two electrons in the inner mitochondrial membrane. Thus, NADH dehydrogenase creates a high H+ ion concentration across the electrochemical gradient.
Co-Q
Coenzyme-Q or “Ubiquinone” connects complex I and II. Ubiquinone is a lipid-soluble complex, which can move freely in the hydrophobic core of the mitochondrial membrane. Q reduces into QH2 and delivers its electron to the third complex. Coenzyme-Q receives the electron released from the NADH and FADH2 molecules.
Complex II
It consists of an enzyme, “Succinate dehydrogenase”, and contains iron and succinate. Complex II oxidizes FADH2 into FAD+. Succinate dehydrogenase plus FADH2 directly transfers the electrons to the ETC, bypassing complex I. It does not energize the complex I and produce a few ATPs.
Complex III
Cytochrome-b, Oxidoreductase or complex III consists of Fe-S protein with Rieske centre (2Fe-Fs). In cytochromes, the prosthetic group is heme, carrying electrons. As the electrons pass, the iron is reduced to Fe2+ and oxidized to Fe3+. Therefore, cytochrome-b transfers electrons to the next complex, i.e. cytochrome c.
Cytochrome c
Cytochrome-c also contains Fe-S protein and prosthetic heme group. It only accepts one electron at a time and further transports electrons to the fourth complex.
Complex IV
It is composed of Cytochrome a and a3, which contains two heme groups (one in each). Cytochrome-a3 consists of three copper ions (two CuA and one CuB). The function of complex IV is to hold the oxygen carrier firmly between the iron and copper ions until the reduction of oxygen into a water molecule. Oxygen combines with the two proton molecules and releases water by maintaining the membrane ion potential.
Complex V
It is the protein ion channel consisting of a transmembrane enzyme (ATP-synthase or ATP-synthase complex). Complex V allows the passage of protons from a high to low concentration against the potential gradient. The chemiosmotic passage of the protons results in molecular rotation of the enzyme ATP synthase and thereby causing a release of ATP.
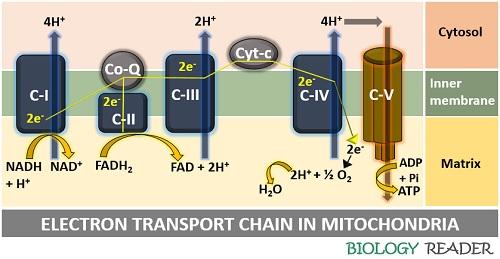
Electron Transport Chain Summary
ETS refers to a system producing energy in the form of ATP via a series of chemical reactions. The ETS is located in the inner membrane of mitochondria, containing electron carrier protein complexes, electron carriers and channel proteins. Electrons pass from one complex to the other by redox reactions.
The free energy during electron transfer is captured as a proton gradient and used up by the ATP synthase to derive ATP. The electron carrier Co-Q receives the electrons formed by the reduction of FADH2 and NADH. Coenzyme-Q reduces into QH2 and passes the electrons to the third protein complex (cyt-b).
Complex III contains a heme group, where the Fe3+ reduces into Fe2+ after accepting the electrons coming from Co-Q. The third complex further transfers the electrons to cyt-c, where Fe3+ reduces into Fe2+ and transfers electrons to the fourth complex.
Complex IV accepts the electrons and transfers them to the oxygen carrier. The oxygen carries the de-energized electrons and combines with the free proton ions in the matrix, and releases waste in the form of water.
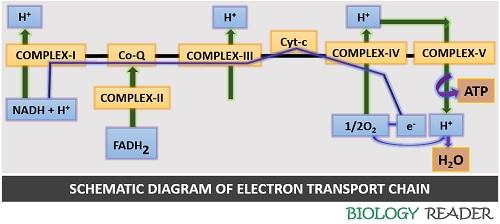
Mechanism of Electron Transport System
The electron transport chain sometimes refers to the “Respiratory chain”, which is the third or final stage of cellular respiration. It requires the presence of oxygen to carry out cellular respiration. The energy is produced during the transfer of electrons from one carrier to the other.
A cell harnesses the energy loss during electron transport to pump protons into the cytosol. It creates a chemiosmotic gradient. A chemiosmotic gradient becomes charged by the potential energy of the electrons. Finally, the potential energy converts into chemical energy (ATP) by the ATP synthase complex.
Thus, the electron transport system is an energy-producing mechanism, which obeys the principle of “Takes energy to make energy”. The ETS possesses a series of redox reactions where the electrons lose energy. The membrane uses the energy loss during the diffusion of protons back into the matrix and creates a high energy molecule, ATP.
Location of ETS
The electron transport system and its protein complexes, along with the ATP synthase channel protein, are located in the inner mitochondrial membrane. In a diagram, we could see the site of the electron transport chain, which is present in between the cytosol and matrix.
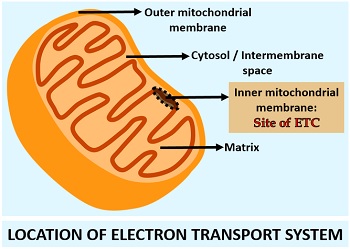
There are four large protein complexes in the electron transport chain, which mediate the transfer of electrons. In addition to protein complexes, there are individual electron carriers present like Co-Q and Cyt-C.
Both coenzyme-Q and cytochrome-C are diffusible electron carriers, which can travel within the membrane. Besides this, there is one ion channel protein (ATP-synthase) that mediates the transport of protons down the concentration gradient by generating ATP.
Equation of ETC
The overall reaction in the electron transport chain can be equated in a way given in a picture. In the electron transport chain, per molecule of glucose can produce 34 molecules of ATP, as given in the equation below:
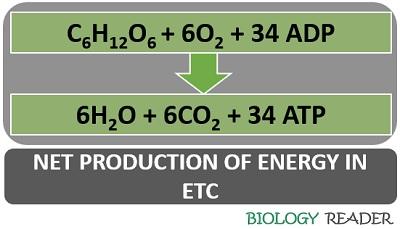
Thus, the net production of energy in the electron transport chain is 34 ATP molecules.