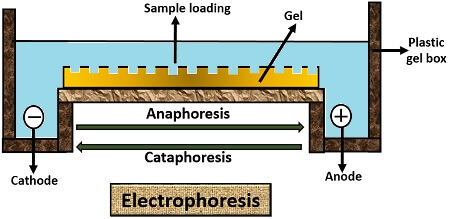
Electrophoresis separates the biomolecules like proteins, nucleic acids, amino acids etc. according to their size, shape, chemical charge etc. under the control of the electric field. Under the influence of the electric field, a positively charged molecule (Anion) will move towards the negatively charged electrode, i.e. Cation.
The negatively charged molecule (Cation) moves towards the positively charged electrode, i.e. Anion. Movement of positively charged ion towards negatively charged electrode refers to “Cataphoresis”. And, the movement of negatively charged ion towards the positively charged electrode refers to “Anaphoresis”.
In this context, we will talk over the discoveries that have led to the introduction of an electrophoresis technique as well as definition, working, factors influencing and different types of electrophoresis methods.
Content: Electrophoresis
Definition of Electrophoresis
Electrophoresis is a mechanical process used for both analytical and preparative measures. It makes the use of substrates or matrices like agarose, cellulose acetate, polyacrylamide. It is used for the analysis and separation of biomolecules like amino acids, proteins, nucleic acids, nucleotides. Based on the net charge, molecular size, shape and binding affinity properties, the molecules can be separated under the force of the electric field through the formation of different bands.
History
Several discoveries have been made to explain the theory of electrophoresis, which are explained below:
| Year | Discovery in the electrophoresis technique |
|---|---|
| 1807 | Movement of clay particles were first observed by the scientist Ferdinand Frederic Reuss who gave the theory of electrophoresis. |
| 1934-1937 | Then apparatus was developed known as Tiseleus apparatus named after the scientist Tiseleus for the analysis of colloidal mixtures. |
| 1940-50 | Zone electrophoresis techniques were then introduced, where filter paper, capillary tubes were used as carrier matrix for the movement of molecules. |
| 1955 | In this year, Oliver Smithies introduced agarose gel and polyacrylamide gels as a substrate for the separation of biomolecules. |
| 1960's | A.L. Shapiro, J.V. Maizel developed relationship between the molecular weight and migration of proteins. |
| 1975 | Farrell and J.Klore developed 2-D electrophoresis. |
| 1981 | Electrophoresis of amino acid was carried out by the two scientist J.W. Jorgensen and K.D. Lukas |
| 1990 | Matrix developed for the DNA separation at high resolution by the B.L. Karger and his group. |
| 2000- now | Many high resolution electrophoresis methods have developed for both analytical and preparative measures. |
Working
The working principle of electrophoresis is based upon the molecule, ions and colloidal particles separation, which are generally suspended in the matrix by applying an electric field. The electric field allows the migration of the positively charged molecule towards the cathode and the migration of negatively charged molecule towards the anode. Therefore, electrophoresis is the electrokinetic phenomenon, in which the charged biomolecules moves under the force of an electric field.
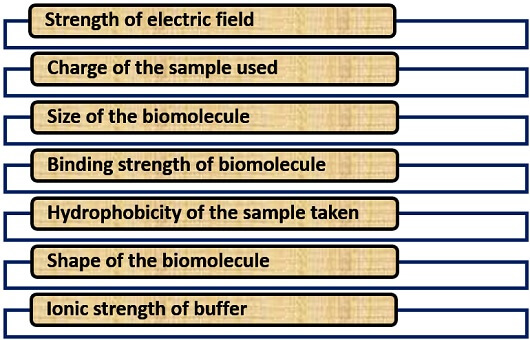
Factors Affecting
Many factors influence the process of electrophoresis like molecular charge, size, a shape of the biomolecule, and the binding affinity of biomolecule etc.
Principle and Mathematics
It depends upon the Coulomb’s law, which is simply the experience of force by the particles or ions under an electric field for electrophoretic mobility. There are two forces Electric force and drag force For the electrophoretic mobility. Thus, the electric forces and drag forces aid for the mobility of ions. As the electric field is the force per unit charge, so it can be expressed as:
E = Fe/q
- F= Force experienced by ion.
- q= Charge
Fe = E X q ………… equation 1
This kind of force, i.e. Fe, is due to an electric field.
Fd = f X V ………… equation 2
This kind of force i.e. Fd is due to Friction.
Fe and Fd must be zero for electrophoretic mobility. By combining both the equations 1 and 2, we will get:
q X E = f X V
q/f = V/E = µ
- µ: Electrophoretic mobility
V = q X E/ f
- V: Velocity or movement of ions in electrophoresis
Types of Electrophoresis
The method of electrophoresis is subdivided into the following types that are explained below.
Paper Electrophoresis
Paper electrophoresis makes the use of chromatography paper that has a different adsorption capacity for the different molecules. To perform this kind of electrophoresis, there are several steps that we need to follow:
- First, moisten the filter paper in the buffer solution.
- After this, immerse both the ends of filter paper in a buffer solution.
- Then, place the sample place at the centre and pass the electric current to facilitate the migration of molecules to their respective poles depending upon their charge.
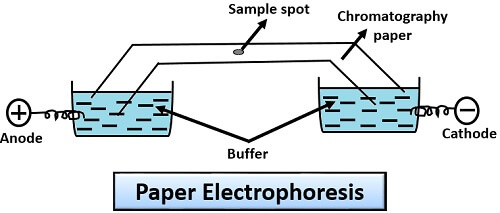
Application: Paper electrophoresis is used for the analysis of proteins like casein, serum, albumin, myosin etc.
Cellulose Acetate Electrophoresis
It was developed by Kohn in 1958. Biological acetate membrane filter used in this method, instead of normal chromatography paper. Cellulose acetate electrophoresis is more or less very similar to the paper electrophoresis. It involves the following steps:
- First, moisten the cellulose acetate filter paper in the buffer solution.
- After that, immerse both the ends of cellulose acetate filter paper in a buffer solution.
- Then, place the sample at the one end and pass electric current to allow the migration of charged molecules to their respective poles relative to their size and charge.
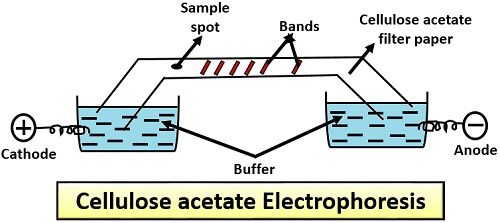
Application: It is used in the separation of proteins like glycoprotein, haemoglobin and lipid-protein molecule (lipoprotein).
Capillary Electrophoresis
This type of electrophoresis makes the use of a capillary tube or narrow bore tube. For its operation, we need to follow the given protocol:
- Firstly, immerse the ends of the capillary tube into the inlet and outlet vials.
- Fill the inlet and outlet vials with an electrolytic solution.
- Connect the electrode to the high power supply up to 5-30 kV.
- Then, inject the sample via a hollow capillary tube through the electrokinetic energy.
- Under the influence of the electric field, different zones of analyte or sample will migrate towards the outlet side.
- These bands are then detected directly by a capillary wall.
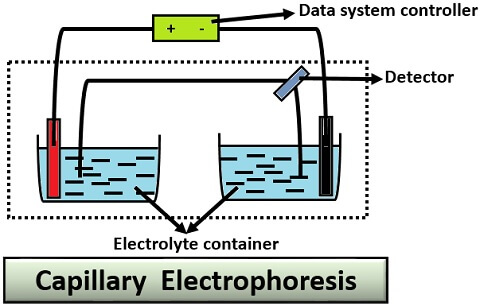
Application: Capillary electrophoresis is used for the analysis of food, pharmaceutical product and environmental pollutants.
Gel Electrophoresis
It makes the use of gel as a support matrix. Among all the electrophoresis methods, it is the most popular and commonly used method for both analytical and preparative processes.
Principle: In this porous gel matrix is used, which consists of the cross-linked polymer network. Through this network, molecules of different size, charge and shape can pass through. Its working relies upon the fact that negatively charged molecule will attract towards the positive end and vice versa by travelling through the pores of the gel matrix. After the migration, bands will appear on the gel matrix at different levels those which lag behind will be the heavy molecules and those which moves faster are lighter molecules.
Types: Gel electrophoresis is subdivided into the following two types:
- Horizontal (Agarose gel electrophoresis)
- Vertical (SDS-PAGE)
Agarose Gel Electrophoresis
Its operation involves the following steps:
- First, take agarose into the water to make the slurry or to dissolve the agarose.
- Cool the agarose solution, and then transfer it to the casting tray containing comb.
- Add electrophoresis buffer (Tris-acetate EDTA buffer) to cover the gel up to 1mm.
- Then, take the DNA sample.
- Add the desired amount of 6X gel loading buffer.
- After that, take out the comb and slowly load the sample mixture containing tracking dye (Bromophenol blue) into the wells through the micropipette.
- Close the lid of the gel tank, and turn on the power supply so that DNA can migrate towards the positive electrode.
- Run the gel, until the bromophenol blue migrates on an appropriate distance through a gel.
- At last, place the gel tray under a UV transilluminator, to see the orange-red coloured DNA bands.
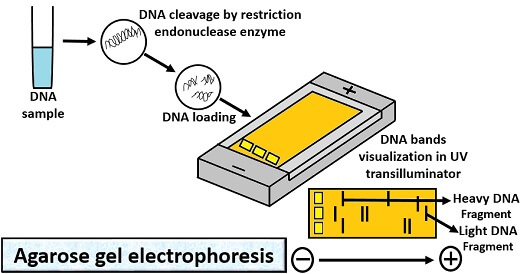
Application: Agarose gel electrophoresis is used for the isolation of nucleic acid especially DNA.
SDS-PAGE
It stands for Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis and its operation include the following steps:
- First, add the resolving gel between the two glass plates of the casting frame.
- Then, place a comb on the glass plates leaving 1cm space.
- Add isopropanol on the top of the gel.
- After the solidification of resolving gel, remove the isopropanol using filter paper.
- Load the stacking gel on the top of the glass plates.
- Then, place a comb and solidify the stacking gel for desired time.
- After solidification of stacking gel, remove the comb to form wells.
- Place a ladder into the extreme right and place the protein sample with a tracking dye (Bromophenol blue) into the other wells, by using a micropipette.
- Then turn on the voltage supply, so that tracking dye can cross the gel by forming different bands.
- After the completion of electrophoresis, take out the gel and rinse it with deionized water 4-5 times to remove SDS and buffer.
- Then, dip the gel in the Coomassie blue stain which is a staining buffer, stains the invisible protein bands after a few hours.
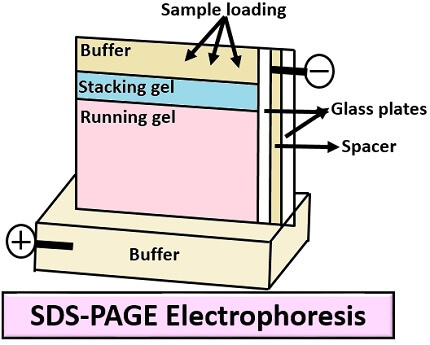
Application: SDS-PAGE is widely used for the isolation or separation of protein.