ELISA test stands for Enzyme–Linked Immunosorbent Assay. It is a type of serological test and immunoassay technique. In the ELISA test, an enzyme links to the antibodies particularly to detect the presence of proteins like antigens. The ELISA method was evolved from the RIA technique in the 1960s.
Therefore, the ELISA technique is more or less similar to Radio Immunoassay Antigen (RIA), in which the antigen is radiolabeled. By the increasing concern over environmental pollution, the ELISA technique has been introduced instead of radioisotopes. Therefore, ELISA is a widely used method for the detection or diagnosis of bacterial and viral infections.
Nowadays, ELISA kits have also been developed that are most frequently used in many laboratories. In this context, we will study the definition, principle, types, advantages and disadvantages of the ELISA test along with its qualitative and quantitative measure.
Content: ELISA Test
Meaning of ELISA
Enzyme-linked immunosorbent assay is the immunological reaction, which is performed on a solid phase, i.e. a microtitre plate that uses enzymes to label the antibodies and indirectly measures the antigens or antibodies concentration in the test sample both qualitatively and quantitatively.
Principle of ELISA Test
ELISA is the immunoassay technique which comes under the category of Labelled immunoassay. Immunoassay depends upon the interaction between an antigen (Ag) and antibody (Ab), which forms the Ag-Ab complex called immunocomplex. ELISA is used to detect the presence and to quantify specific antigens or antibodies. ELISA being an immunoassay method is very sensitive and specific in nature whereby the specific antigens or antibodies bind to their homologous antibodies and antigens, respectively.
Overview of ELISA Test
ELISA is performed on a Microtitre plate. A microtitre plate is made up of polystyrene, and it consists of 96 shallow wells or sometimes more than that. For instance, the proteins, i.e. antigens, are immobilized on the solid surface of the well to detect the presence of antigen. Then, the enzyme-linked antibodies are introduced into the well. After that, the result could be interpreted in the following two ways:
- Positive ELISA: If the enzyme-linked antibodies bind to the specific antigens, then this will give a positive result for the ELISA. We can neither see the interaction of antigen and antibody nor the presence of antigen or antibody. Therefore, the colourless substrate is added to the wells where it reacts with the enzymes that are linked to the antigen-antibody complex and later release some signals in the form of colour change. Hence, a positive result is indicated by a colour change, and higher is the concentration of antigen higher is the signals produced by the substrate.
- Negative ELISA: If the enzyme-linked antibodies do not bind to the antigens or there is no such interaction of antigen and antibody, then it gives the negative result for ELISA. Therefore, in negative ELISA, we can neither see any colour change by the addition of a substrate nor any bonding between the Ags and Abs.
Therefore, we can say an ELISA is basically a colourimetric assay, which is detected by any colour change in the wells to conclude the presence or absence of antigens finally.
Types of ELISA Test
ELISA is mainly of four types, namely direct, sandwich, indirect and competitive ELISA.
Direct ELISA
It includes the following steps:
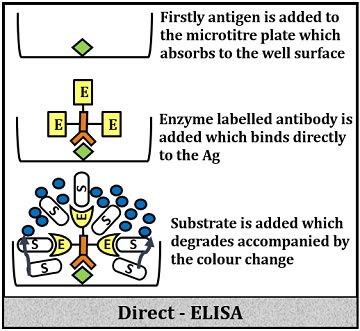
- Firstly, add the buffered protein sample into the wells of the microtitre plate.
- Then, add surface blocking or non-reacting proteins like BSA (Bovine Serum Albumin), Casein etc. to block the bottom surface to cover the extra space and to prevent false binding or false signalling.
- Add specific primary antibodies conjugated with the enzymes, which will directly stick to the desired protein or antigen.
- Then, flood the washing buffer to remove the unbound proteins.
- Add substrate to the above complex, which will further react with the enzymes and produce significant signals in the form of colour change.
Indirect ELISA
It includes the following steps:
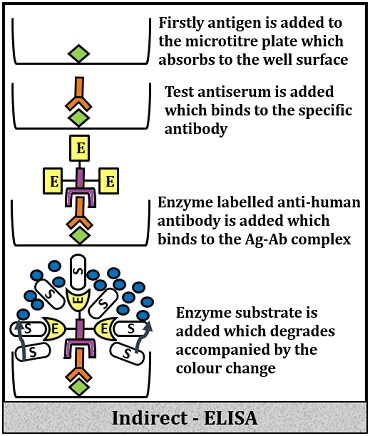
- Firstly, add buffer protein sample into the wells of the microtitre plate.
- Then, add surface blocking or non-reacting protein like BSA (Bovine Serum Albumin), Casein etc. to block the bottom surface to cover the extra space and to prevent false binding or false signalling.
- Add specific primary antibodies that will bind to the protein of interest.
- Then, add the washing buffer to remove the unbound proteins.
- Add secondary antibody conjugated with enzymes, which will bind to the antigen-antibody complex.
- Again, add washing buffer to remove the unbound secondary antibodies.
- Add substrate to the above complex, which will further react with the enzymes and produce significant signals in the form of colour change.
Sandwich ELISA
It includes the following steps:
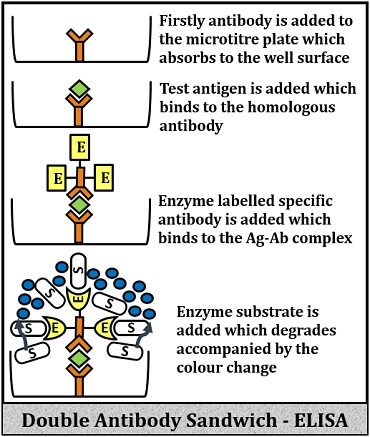
- In this technique, add the antiserum to the wells of the microtitre plate.
- After that, the antibodies that are present in the antiserum will adhere to the well- surface.
- Then, add the test antigens, which later couples with the homologous antibodies.
- Add enzymes labelled specific antibodies, which will eventually bind with the antigens that are coupled with the antibodies adhered to the well surface. This reaction results in the formation of antibody-antigen-antibody complex, which resembles a sandwich.
- Add the enzyme-substrate to the above complex, after which it will react with the enzymes and degrade the substrate molecules by the enzymatic activity.
Competitive ELISA
It includes the following steps:
- Firstly, coat the bottom of the well that will capture the antibody.
- Then, add surface blocking proteins with BSA or detergents.
- Mix the sample with enzyme-conjugated proteins.
- After that, add the mixture to the well-containing antibodies that are adhered to its surface.
- Wash the wells with washing buffer to remove unbound proteins.
- Add substrate to the above complex, which will react with the enzymes and indicate the presence of protein or antigen in the given sample by giving a significant colour.
Qualitative Measure of ELISA
For the qualitative measure or to detect the presence of antigens or antibodies, the enzymes are used. It is very much clear from the name itself that the Enzyme-Linked ImmunoSorbent Assay makes the use of an enzyme system. In the ELISA method, the most commonly used enzymes are as follows:
- HRP: It stands for Horseradish peroxidase. HRP uses the following substrates viz. OPD, TMB, ABTS etc.
- OPD gives Amber colour to the solution.
- TMB gives Blue colour to the solution.
- ABTS gives a green colour to the solution.
- AP: It stands for Alkaline phosphatase. AP makes the use for PNPP substrate that gives a yellow colour to the solution.
Therefore, in ELISA, positive and negative results are made based on the colour change. If the solution remains colourless even by the addition of substrate, then it results in negative ELISA. If the colour of the solution changes from colourless, then it will indicate the presence of analyte or protein of interest.
Quantitative Measure of ELISA
For the quantitative measure of ELISA, there are some key points that we have to remember, like:
- Sample: The sample is added either in duplicates or triplicates. Suppose, we have 40 samples, then the sample is added twice, and if we have 20 samples, then it is added thrice. Therefore, the addition of a sample depends upon the number of samples we have. Triplicates of a sample are considered to be better than the duplicates because it reduces the chances of error in the result.
- Standard: To prepare standard of the given sample, the sample is diluted in a series of 0.5, 1, 2, 4, 8, 16, and 32.
- Positive control: Positive control is the known concentration of the sample.
- Blank samples: It contains everything as protein.
- Standard-curve: The standard curve is prepared by diluting the concentrated samples to find the unknown concentration of the protein by plotting a graph between adsorption and concentration.
Advantages of ELISA Test
- ELISA is the qualitative and quantitative assay, i.e. it not only detects the presence of the analyte but also detects the concentration or quantity of the analyte.
- It can screen a large number of samples in a single take.
- ELISA can be easily automated.
Disadvantages of ELISA Test
- ELISA does not give any information about the biochemical properties of the analyte such as molecular weight, distribution among the living organisms etc.
- To know the molecular weight and more information about the analyte, a technique like Western blotting is generally employed.
- ELISA is a very sensitive method to perform, which requires more precautions while experimenting.
Therefore, the ELISA has both advantages along with some disadvantages. Inspite of this, it is an advantageous technique in medical science and research.