Alum is used to purify water or to remove the hardness of the water. Its usage has been popular from a long ago till now because it is a readily available and inexpensive way to clean turbid water.
The use of alum together with filtration is one of the standard practices in conventional water treatment methods. Nowadays, people being more concerned about water sanitation use a candle, aqua, RO filters, etc., to get the filtered water.
So, people living in rural and urban areas apply all possible ways to make the raw water potable or safe for consumption. Alum works as a flocculant that removes the pollutants responsible for the water turbidity.
This content will give you insights into the alum’s meaning, chemical formula and properties. Also, it will explain why we use alum, how alum purifies the muddy water, and how you can apply alum to purify the raw water.
Content: How to Use Alum to Purify Water?
Alum Meaning
Alum is a generic term for a group of hydrated double salts produced from a combination of water, aluminium and group IA metal sulfates. It is an inorganic compound with an empirical formula of AB(SO4)2·12H2O. Alum is generally available in its crystalline form or most often as a powder.

Potash alum or fitkari is used to clean the water as the potassium sulfate dodecahydrate. It is a colourless soluble hydrated double sulphate of aluminium and potassium with a chemical formula K2SO4.Al2(SO4)3. 24H2O. Its physical and chemical properties are given hereunder.
Physical Properties
- Physical State: Hygroscopic solid
- Appearance: White crystals
- Colour: Colourless
- Taste: Styptic
- Solubility: Partially soluble in water or insoluble in ethanol
Chemical Properties
- When potash alum is burnt, it turns red and is called the burnt alum.
- On overheating, it losses 12 water molecules and gets converted into [KAI (SO4)2].
How Alum Purifies the Muddy Water?
Muddy or turbid water is a colloidal solution containing various suspended particles. The colloidal particles in the water absorb ions and get the same electric charge (either positive or negative). Thus, particles repel one another and remain dispersed in turbid water.
So, to neutralize the electrical charges on the suspended particles, a coagulant is added. The purpose of adding coagulant is to prevent repulsion between the dispersed particles.
The amount of coagulant should be added considering the zeta potential (amount of repulsive force acting on the suspended particles in the water).
The oppositely charged coagulants attract the colloidal particles, resulting in a neutral charge. As a result, the particles no longer repel each other.
Next, Van der Waal’s forces will cause the particles to drift together, forming a precipitate. Once enough particles merge together, they become floc and will sink to the bottom.
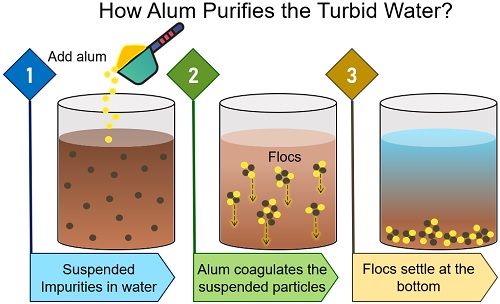
So, potash alum, potassium aluminium sulfate or fitkari cleans the water by neutralizing the electrical charges on the clay, silt and fine suspended particles in the water.
- Potash aluminium sulfate in the muddy water reacts with the suspended bicarbonate alkalinities, clay or silt particles to form aggregates or flocs.
- Then, other finer impurities in the water drift towards these flocs.
- Eventually, flocs become large enough and sink to the bottom of a container.
- Water over the settled flocs or sediment is almost clean or safe to use for drinking and cooking purposes.
Thus, potash alum destabilizes the colloidal particles in the water through coagulation.
How to Purify Muddy Water?
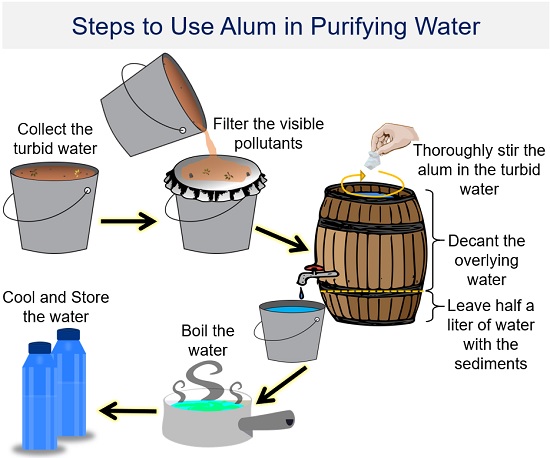
1. Collection of water: First, collect the turbid water in a bucket.
2. Filtration of water: If you notice any floating material, use a fine cloth to separate it from the raw water.
3. Addition of alum: Add alum to the turbid water and stir it well.
4. Leave the bucket: Till the time you leave the bucket, the potash alum coagulates with the mud particles to form large aggregates called “Flocs”. Later, these flocs settle down at the bottom.
5. Decantation: Drain the clean water into another bucket without disturbing the sediment.
6. Disinfection: Water decanted from the bucket may still contain finer particles and pathogenic microorganisms, so it must be disinfected. So, you can disinfect your water by simply boiling or adding chlorine to the water system.
- The addition of chlorine to the water system damages the cell membrane of the microorganisms, thereby disrupting cell respiration and DNA activity. Only 4 mg/liter of chlorine is regarded safe for human consumption. But, applying chlorine to the water system can somehow cause long-term side effects.
- Boiling water is the safest method to kill pathogenic bacteria, viruses and protozoa. You must boil the water for at least one minute until the large bubbles rise quickly to the water surface. Then, cool the water at room temperature and store it in clean containers with covers.
Importance of Alum in Water Treatment
Potash alum has widespread use around the world. But do you know why it is so popular in water treatment? Following are the factors that make it suitable to purify turbid water.
Separates Pollutants Causing Water Turbidity
Alum removes clay, silt and other suspended matter associated with the brown colour of the water, especially during monsoons. Such pollutants make the water turbid by turning the water opaque, cloudy or hazy.
The heavy particles of alum in water coagulate the suspended clay particles to form flocs. So, the silty brown-coloured water turns clear as the flocs settle down at the bottom of the earthen pots, buckets or any containers you use.
Lowers the Hardness of Water
It also removes the hardness of water caused due to the high concentration of Ca and Mg mineral salts in the form of bicarbonates, sulphates and chlorides.
Alum react with bicarbonate alkalinities in the water and forms a viscous precipitate. Later, the precipitate sink to the bottom and the overlying water can be separated.
Reduces Phosphorous Concentration
Alum treatment proves to be good in reducing the amount of phosphorous from the lakes. It started in the early 1970s. Phosphorous in water reservoirs deteriorate the water quality by supporting algal growth.
The application of alum into the lakes controls the phosphorus release from the lake bottom sediments. Through specialized equipment, alum is carefully placed in the lake.
Later, a liquid alum produces a precipitated mass of aluminium hydroxide called floc. Aluminium hydroxide plus phosphorus forms an insoluble aluminium phosphate compound that the algae cannot utilize.
The floc layer makes the phosphorous unavailable to the algae and prevents the phosphorus from entering the overlying clear water. So, alum can reduce the growth of the nuisance algal bloom.