Nitrification is one of the crucial steps in the nitrogen cycle that occurs in soil. It is an aerobic process that involves two successive oxidation reactions, in which the ammonia first oxidizes into nitrites, and then nitrites get oxidized into nitrates.
It occurs in the soil and includes members of autotrophic bacteria and archaea. The soil microorganisms that facilitate the interconversion of soil ammonia into the inorganic nitrates or participate in the process of nitrification are called “Nitrifying bacteria”.
The energy yield in nitrification is quite low, and the process is relatively slower than the other processes occurring in the nitrogen cycle. Thus, nitrification is an oxidative process whereby ammonia oxidizes into nitrate by producing nitrite as an intermediate. This post describes the definition, steps, key points and factors affecting nitrification.
Content: Nitrification
Definition of Nitrification
It refers to the biological process in which ammonia (NH3) or ammonium (NH4+) primarily converts into nitrites (NO2–) and then into nitrates (NO3–). Its mechanism involves two distinct energy-producing reactions, which is further used to fix carbon dioxide.
- Oxidation of ammonia to nitrite: It is the first reaction where the nitrosifying or ammonia-oxidizing bacteria catalyzes the transformation of NH3 to NO2–. This reaction is metabolically inefficient, which requires 34 moles of NH3 to fix 1 mole of CO2.
- Oxidation of nitrite to nitrate: It is the second reaction where the nitrite-oxidizing organisms catalyze the transformation of NO2– to NO3–. This reaction is even less efficient, which needs around 100 moles of NO2– to fix 1 mole of CO2.
Process
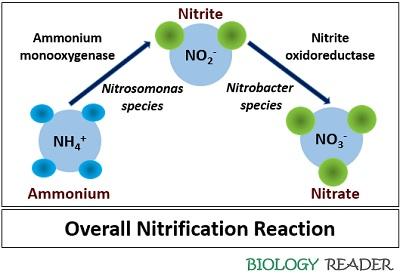
Nitrification is a two-step process mediated by specific groups of soil microorganisms.
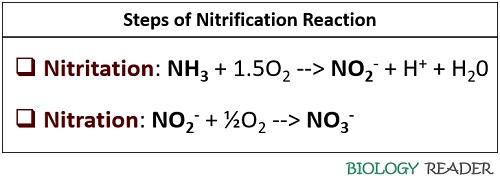
Nitritation: The initial step involves the oxidation of ammonia into nitrogen dioxide or nitrites. Nitrosomonas are the special group of bacteria that participate in nitritation. This reaction uses an enzyme system, i.e. ammonium monooxygenase. The chemical equation for this process is:
NH3 + 1.5O2 –> NO2– + H+ + H20
Nitration: It is the second phase, which facilitates the oxidation of nitrites (NO2–) into nitrates (NO3–). Nitration involves nitrite-oxidizing bacteria like Nitrobacter and Nitrospira species and an enzyme system of nitrite oxidoreductase (NOR) to complete the process. The chemical equation for this reaction is:
NO2– + ½O2 –> NO3–
After these two reactions, an organic form of nitrogen (soil ammonia) converts into an inorganic form of nitrogen (nitrate), which plants can use. The chemical equation for the entire nitrification process is given below:
2NH3 + 3O2 –> 2NO2– + 2H+ + 2H20
Key Points
- It is an aerobic chemoautotrophic process that requires oxygen to oxidize ammonium and nitrite through ammonia-oxidizing and nitrite-oxidizing aerobes, respectively.
- Ammonia-oxidizing bacteria (e.g. Nitrosomonas) are the soil microorganisms that mediate ammonia oxidation reactions.
- Nitrite-oxidizing bacteria (e.g. Nitrobacter) mediate nitrite oxidation reactions.
- Both the nitrifying reactions are thermodynamically favourable but occur slowly.
- The efficiency or rate of nitrification is limited due to the low metabolic efficiency and slow growth rate of nitrifying organisms.
- Most nitrifying bacteria are autotrophic, while few are heterotrophic.
Factors Affecting Nitrification
The efficiency of nitrification reaction depends upon the various environmental factors that include:
Soil pH
The nitrifying microorganisms are pH sensitive and require pH between 6.6 to 8.0. In addition, the microflora participating in nitrification obtain an adequate supply of calcium ions, dihydrogen phosphate and other essential elements in the soil environment.
- pH above 8.0 to 0.9 accelerates the nitrification rate.
- pH above 6.0 slows down nitrification, and pH below 4.5 inhibits nitrification.
Although, few nitrifying heterotrophic fungi can directly fix the organic nitrogen to nitrate without oxidizing ammonia, as they are resistant to the soil’s acidic pH due to slime production.
Soil Oxygen
Nitrifying microorganisms are obligate aerobes, which need oxygen to produce inorganic nitrogen like nitrate ions. Nitrification occurs at the optimum aeration porosity between 50-60% of the total soil porosity. The nitrification process in aggregated soil mainly constricts to the outer region due to the speedy diffusion of oxygen into the atmosphere.
Soil Moisture
Nitrification mainly occurs at an optimum soil moisture potential of −0.3 to −1.6 MPa. In waterlogged or saturated conditions, nitrifying bacteria are not feasible to carry out nitrification. During water stress, autotrophic bacteria are more likely affected in comparison to fungal nitrifiers.
Soil Temperature
The optimum range of temperature that favours the growth of nitrifying bacteria lies between 28°C and 36°C. In forest soil, nitrification occurs at the temperature of 0°C due to the predominance of low-temperature-adaptive nitrifying soil fungi. The temperature fluctuations in the soil also affect the rate of nitrification. Nitrification efficiently occurs during the time of spring and fall.
Soil Organic Matter
Nitrification includes the autotrophic nitrifying organisms that do not need soil organic matter as either a carbon or energy source.
The population of Nitrifying Organisms
Soils may differ in their ability to nitrify NH3 because of the diversified population density of nitrifying organisms. There are three categories of nitrifying organisms.
- The first category includes ammonia-oxidizing organisms (obligate autotrophs) that can fix CO2 using the Calvin cycle.
- The second category involves autotrophic ammonia-oxidizing archaea, which fix CO2 using the 3-HP (hydroxypropionate pathway).
- The third category includes aquatic bacteria of the Planctomycetes phylum.
Cultural Practices
The use of pesticides reduces the efficiency of nitrification due to their toxic effect on soil microbiota. In contrast, tillage increases the rate of nitrification by improving soil aeration.
Crop Type
Ploughed grasslands produce adequate inorganic nitrate. Brachiaria humidicola and Azadirachta indica produce root exudates containing brachialactone, showing a 60%–90% inhibitory effect on nitrification.
Significance
The nitrification process plays a significant role in agriculture (where it can facilitate nitrate leaching and determine the availability of fertilizer nitrogen) and in wastewater treatment systems (where it prevents groundwater contamination by removing excess nitrogen).