The difference between ATP and ADP is primarily due to the three factors like their energy state, the number of phosphate groups and the hydrolysis process. ATP is comparatively a high energy molecule than the ADP.
There are three phosphate groups in ATP, while two in ADP. A cell needs energy to perform different tasks, for which it hydrolyzes ATP into ADP and later into AMP. Both ATP and ADP molecules are the two universal power sources, which mediate various biological or cellular functions.
This post describes the definition, structure and concept of ATP and ADP, along with the comparison chart. In addition, the key differences and similarities between the two have also been explained.
Content: ATP Vs ADP
Comparison Chart
| Properties | ATP | ADP |
|---|---|---|
| Full form | Adenosine tri-phosphate | Adenosine di-phosphate |
| Common name | Adenosine 5’-triphosphate | Adenosine 5’-diphosphate |
| Alternative name | It has no such alternative names | Adenosine pyrophosphate |
| Molecular formula | C10H16N5O13P3 | C10H15N5O10P2 |
| Molar mass | 507.18 g/mol | 427.2 g/mol |
| Formulation | Solid | Crystalline solid |
| Number of phosphate groups | Three | Two |
| Form of | Potential energy | Kinetic energy |
| Energy state | Higher | Comparatively lower |
| Stability | Relatively unstable | Comparatively stable |
| Hydrolytic reaction | The reaction of ATP with water causes the formation of ADP and release of energy by the removal of one phosphate group | The reaction of ADP with water causes the formation of AMP and release of energy by the removal of one more phosphate group |
| Conversion reaction | Endergonic reaction | Exergonic reaction |
| Functions | Helps in active transport, building of molecules, cellular functions like muscle movement etc. | Helps in catabolism reactions, in the activation of platelets etc. |
Definition of ATP
ATP is an acronym for the term Adenosine tri-phosphate, which merely refers to the high energy organic biomolecule that drives many biological processes by donating its high energy phosphate molecule. The structure of adenosine tri-phosphate includes three distinct groups:
- Adenine (nucleotide base)
- Ribose (pentose sugar)
- Triphosphate (phosphoryl group)
The active form of adenosine tri-phosphate contains a combination of ATP molecules with Mg2+ or Mn2+ ions. It serves as the energy source necessary for all the life forms, which fuels different cells to promote specific functions. ATP mediates intracellular energy transfer.
Definition of ADP
ADP is an acronym for Adenosine di-phosphate, which merely refers to the comparatively low energy organic biomolecule that mediates energy flow by donating its high energy phosphate molecule. The structure of adenosine di-phosphate includes three distinct groups:
- Adenine (nucleotide base)
- Ribose (pentose sugar)
- Diphosphate (phosphoryl group).
ADP also mediates the intracellular energy flow. It is also known as adenosine pyrophosphate. ADP refers to the product formed by the ATP dephosphorylation via an ATP synthase.
Structure of Adenosine triphosphate
- Adenine: It is the nitrogenous base, whose 9th nitrogen atom attaches with the 1st carbon atom of the ribose sugar.
- Ribose sugar: It is the pentose sugar, located intermediary between the adenine and phosphoryl group.
- Phosphate: The phosphoryl group associates with the 5th carbon atom of the ribose sugar. They are three in number and comprises α, β and γ terminal phosphates linked via phosphodiester bond, followed by two phosphoanhydride bonds.
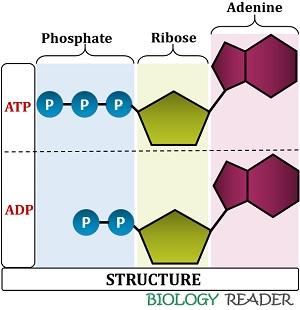
Structure of Adenosine diphosphate
- Adenine: It is the nitrogenous base, whose 9th nitrogen atom is attached with the 1st carbon atom of the ribose sugar.
- Ribose sugar: It is the pentose sugar found intermediary between the adenine and phosphoryl group.
- Phosphate: The phosphoryl group is joined to the 5th carbon atom of the ribose sugar. They are two in number (comprises only α and β phosphate groups) linked via phosphodiester bond, followed by one phosphoanhydride bond.
Examples
We could understand the concept of ATP and ADP by taking the following references:
- Consider ATP as a fully charged battery. When the power of the battery reduces, it indicates the loss of energy. Hence, the reduced form of energy or partially charged battery will be considered as ADP.
- If we have to buy a product, we must need a source of capital or cash. Similarly, a cell must need a particular source of energy to perform specific tasks. Consider ATP as a bank check. To purchase a product, we need to convert the check amount into cash. Therefore, a cell also converts ATP into ADP and energy to do various cellular functions.
Key Differences Between ATP and ADP
- The molecular formula of ATP is C10H16N5O13P3. Hydrolysis causes the elimination of one hydrogen, two oxygen and one phosphate group from the ATP, and the molecular formula of ADP becomes C10H15N5O10P2.
- One of the common distinguishing features between ATP and ADP is the number of phosphate groups present. There are three phosphate groups in ATP and two in ADP.
- ATP can be considered the potential energy, which is basically the stored energy used by a cell to do particular tasks. ADP can be considered kinetic energy, which is necessary for the flow of energy.
- The hydrolysis of ATP and ADP causes the formation of ADP and AMP, respectively. It results in a release of free energy after the removal of one phosphate group.
- The process of ATP formation from ADP is an endergonic reaction (consumes energy), whereas ADP formation from ATP is an exergonic reaction (dissipates energy).
- Functions of ATP promotes active transport, the building of molecules, cellular functions like muscle movement, while ADP helps in catabolic reactions, activation of platelets etc.
Similarities
Despite many differences, they also share some common features:
- Both ATP and ADP are the energy molecules that can drive cellular functions.
- Their structures are common in having an adenine base, a ribose sugar and a phosphate group.
- Both are constantly interconverted and regenerated inside a body.
Conclusion
We can conclude that a cell needs both adenosine triphosphate and adenosine diphosphate as a source of energy to drive many cellular functions like respiration, digestion, muscle movement etc. ATPase enzymatic activity causes a constant interconversion of ATP, and cellular respiration aids in a continuous regeneration of ATP to fulfil the energy requirements of the living cells.