The production of bioethanol is a technique of carbohydrate fermentation by using starch and cellulosic substrates through microbial activity. Substrates like corn, wheat, reed canary grasses etc., are used as a source of carbohydrate in the production of bioethanol. Bioethanol is used as a substitute for fossil fuels.
Currently, bioethanol is used as an additive in combination with petrol. It substituted lead because ethanol functions as an octane enhancer. The combination of petrol (90%) and ethanol (10%) is the most common blend in the fossil fuel industry.
Content: Production of Bioethanol
Definition of Bioethanol
Bioethanol refers to the alcohol produced by the fermentation of starch and lignocellulosic substrates via microorganisms, releasing recyclable carbon dioxide, water and heat. The carbon dioxide released by the combustion of bioethanol can recycle in microalgae production as a carbon source. The generation of bioethanol typically includes four stages of operation:
- Pretreatment of cellulose substrate to produce hemicellulose or lignin.
- Hydrolysis of cellulose to obtain fermentable sugars.
- Fermentation of sugar into ethanol by the biomass.
- Distillation and purification of ethanol.
Important Facts
- The combination of petrol and ethanol reduces the emission of greenhouse gases by oxygenating the fuel mixture.
- Bioethanol is the most favourable substitute for fossil fuels.
- Bioethanol derives from the lignocellulosic biomass serves as an alternative favourable carbon-neutral biofuel.
- There is ongoing research on the development of technologies to produce bioethanol by the fermentation of municipal solid waste, using microalgae, etc.
- The combustion of bioethanol emits carbon dioxide, steam and heat.
- Bioethanol is a clear, colourless, biodegradable liquid possessing low toxicity.
- The mass production of bioethanol was first started in Brazil and the US during the year 1970 by using sugarcane and corn as a carbohydrate source.
- In addition to petrol, bioethanol can also combine with gasoline that also reduces greenhouse gas emissions.
- The market potential of bioethanol is not limited to the use of charging automobiles, gasoline activities and in industries as paints, cosmetics, pharmaceuticals, beverages etc.
- Conventional or advanced biofuel technologies can produce biofuel. Bioethanol from sugar or starchy materials is the traditional method refers as first-generation bioethanol. Bioethanol from lignocellulosic content (wood chips, grasses, crop residues, etc.) is the advanced method known as second-generation bioethanol.
- Production of 0.51 g of bioethanol consumes one gram of glucose.
- Brazil and the US are the primary producers of ethanol in the world, accounting for 35% of global production.
Generations of Bioethanol
There are four generations of bioethanol that uses different feedstocks.
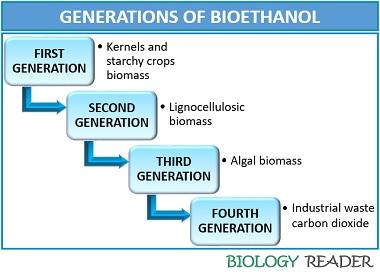
- First-generation bioethanol: It makes the use of kernels and starchy crops biomass like sugar-beet, sugar-cane, wheat, corn etc. The production process requires more land area for the cultivation of crops, due to which the capital cost in the first generation is quite higher. The bioethanol produced from such feedstocks contains a high sugar concentration comparative to the other feedstocks.
- Second-generation bioethanol: It makes the use of lignocellulosic biomass like wood, straw, grass and wastes etc. The production process does not require much capital cost for the maintenance and operation of sophisticated equipment. The feedstock necessary for its production can be grown in poor quality marginal land, which produces low greenhouse gas emissions. Its conversion efficiency is low.
- Third generation bioethanol: It makes the use of algal biomass whose cultivation is easy (can be cultivated on marginal land). Bioethanol derived through the cultivation of algal biomass needs low capital and has high energy density and conversion energy.
- Fourth-generation bioethanol: It makes the use of industrial waste carbon dioxide. The bioethanol produced by this process is considered a carbon-negative biofuel. Further research is still going on.
Bioethanol Production
The production process of bioethanol can be generalized into the following five stages:
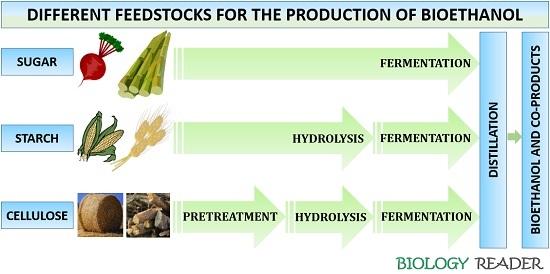
Sample preparation
Bioethanol can be produced by using different substrates like simple sugar (cane sugar), starch (corn) and lignocellulosic (wheat straw) biomass. For the sample preparation, it is essential to prevent pre-fermentation and bacterial contamination once the feedstock is delivered to the ethanol plant. The raw materials must include washing, cutting and drying stages for upto three days. Then transfer the mixture to the milling unit, and store the dried biomass inside a sealed bag at room temperature.
Pretreatment
Sources containing simple sugars and starch does not require pretreatment as the microorganisms or their enzymes can quickly process these during the fermentation process. But the feedstock having lignocellulosic content requires a pretreatment to separate cellulose from lignin and hemicellulose. In the case of lignocellulosic sources like the milled grasses are suspended in 75% anhydrous peroxide, to which NaOH is added to adjust the pH to 11.5. The mixture is then incubated at 35 degrees Celsius and then centrifuged at 250 rpm for 24 hours.
Then, concentrated HCl is added to adjust the pH to 4.8 before hydrolysis. Filtration is not required after the pretreatment. The liquid phase with solubilized hemicellulose and solid phase with the cellulose of the sample is subjected to enzymatic hydrolysis. Thus, the feedstock is first delignified to make cellulose accessible to the hydrolysis step. The pretreatment step makes the use of acid, alkali, organic solvents, heat treatment etc.
Enzyme Hydrolysis
The degradation of starch content like corn, wheat etc., involves the association of amylase enzyme. In contrast, degradation of cellulosic material like wheat straw, grasses etc., consists of the association of cellulase enzyme.
In 10 grams of dried sugarcane, 200 ml of distilled water is added. Then add the above solution into a solution containing 0.5 ml of NaOH at a pH of 4.5. After that, 0.2 µl of enzyme α-amylase is diluted with the phosphate buffer, and the temperature is maintained at 50 degrees Celsius. The mixture is then cooled to 32 degrees Celsius. The hydrolysis of starch requires alpha-amylase and glucoamylase under high-temperature conditions.
The hydrolysis of cellulose involves steam explosion and dilute acid prehydrolysis, followed by enzymatic hydrolysis. To improve the solubilisation of hemicellulose, H2SO4 and CO2 are often added, releasing simple sugars like xylose, glucose etc. As a result of hydrolysis, acetic acid, H2SO4, inhibitors are produced, which must be removed. Second stage hydrolysis, the cellulose biomass into glucose by concentrated or dilute acid or cellulase enzyme.
Fermentation
It is a biological process that mediates the conversion of simple sugars into ethanol via the association of microorganisms like yeasts, bacteria etc. Saccharomyces cerevisiae and Escherichia coli are the most common organisms that are being used in the fermentation stage. S. cerevisiae can ferment the simple sugar into ethanol and carbon dioxide at a temperature of 37 degrees Celsius at a pH ranging from 3-5.
But in the case of starch and cellulose, the fermentation process can carry out in two possible ways. The enzyme hydrolysis and the fermentation can occur separately and simultaneously by a method known as separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF). The SSF process yields much ethanol with reduced formation of inhibitory end products comparative to the SHF method.
Distillation
After fermentation, the recovery of ethanol from the whole suspension or fermentation broth refers to the process of distillation. This stage filters out the fermentation broth to extract bioethanol from the remaining residue through Whatsmann filter paper. Then boil the liquid mixture of water and ethanol.
As the water has a high boiling pressure (100 degrees Celsius) than the ethanol having 78.3 degrees Celsius, so the ethanol will get separated from the solution mixture as vapours via the rotary evaporator. During this process, all the co-products and impurities or residues, enzymes, ash etc., settle down at the bottom of the distillation column.
Advantages
- Bioethanol has a high octane number
- It has a low boiling point.
- It also comprises a high heat of vaporization.
- Water discharged from the bioethanol production plant is environmentally neutral that does not harm the ecology.
- It improves energy safety and the operation of transport facilities.
Applications
- Used in petrol engines as an alternative for gasoline.
- It is suitable for residential use as it does not require a chimney.
- It is fuel for power generation and cogeneration systems.
- Bioethanol is also used as a feedstock in the chemical industry.
Conclusion
Finally, we can conclude that bioethanol is not only used as a transportation fuel but also as a fuel additive. The production of ethanol from edible and inedible sources provides opportunities to produce alternative transportation fuels. Bioethanol is a fossil fuel that reduces greenhouse gases emission and is cost-effective, i.e. it is beneficial from an economic and environmental point of view. The biofuel industry needs to combine integrated production technology and must produce value-added by-products.