The qualitative analysis of amino acids is characterized by the colour change, precipitation, ring formation etc. based on which the amino acids can be detected and classified. The colour change is due to the change in the moiety or the structural configuration, in which the functional group of amino acid react with the specific reagent to give specific results.
Amino acids are basic components of the proteins that are produced after protein hydrolysis. Amino acids possess chirality, i.e. they exist as enantiomers (two optically active asymmetric forms). In this context, you will get to know the meaning and different qualitative methods of amino acid.
Content: Qualitative Analysis of Amino Acids
Definition of Qualitative Analysis of Amino Acids
Qualitative analysis of amino acids is defined as the analytical method, which detects the presence or absence of amino acids in a solution based on the qualitative measures (like colour change, precipitation reaction etc.). We can determine the type of amino acids by the reaction between the functional groups of amino acids and the chemical reagents, which ultimately gives a characteristic colour to the sample.
Meaning of Amino Acid
Amino acids are the basic building blocks of protein. The amino acid structure consists of an amino group (-NH2), a carboxylic group (-COOH) and R-group or side chain.
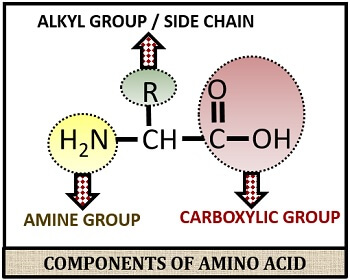
The side chain differs among all 20 different naturally occurring amino acids. The 20 naturally occurring amino acids are the essential amino acids, which are broadly classified into three types:
- Polar amino acids: They possess a spectrum of functional groups. A -COOH and -NH2 group functions as hydrogen bond acceptor and hydrogen bond donor, respectively.
- Non-polar amino acids: They possess either aliphatic or aromatic R-groups and hydrophilic in nature.
- Acidic amino acids: These comprise carboxylic acid on their side chain.
- Basic amino acids: These comprise basic side chain that accepts a proton.
The melting point of an amino acid is about 200 degrees Celsius. All amino acids’ net charge becomes zero at a neutral pH or at isoelectric point (pI), where they exist as zwitterions. At an isoelectric point, the amino acid carries both positive and negative charge, or it will neither move to cathode nor anode, even under the electric field’s influence. Isoelectric point or pI is different for different amino acids. Except for glycine, all the amino acids have an asymmetric C-atom (carbon linked to 4 different groups), which shows optical activity to rotate a polarized light either to left or right.
Methods for Qualitative Analysis of Amino Acids
Several methods are used for the qualitative analysis of amino acids, which includes the following tests that are given below:
Ninhydrin Test
This test is used for the detection of all α-L-amino acids. Ninhydrin test is based upon the principle that makes the use of reagent ninhydrin. The amino acid reacts with the chemical reagent ninhydrin to form an intermediate hydrindantin. Hydrindantin further reacts with ninhydrin and ammonia to form a blue-purple pigment or Ruhemann’s purple compound called diketohydrin.
Therefore, the amino acid undergoes degradation through the series of chemical reaction to give specific results. Other than amino acids, imino acids like proline, hydroxyproline also reacts with the ninhydrin and gives a yellow coloured compound. Amines also react positively by reacting with the ninhydrin reagent and gives a blue colour.
Method:
- Prepare a 1 ml solution of the given sample.
- Then, add a few drops of ninhydrin solution.
- Boil the solution for 2 minutes and then cool the content.
Observation: Observe the tubes for the appearance of any colour change.
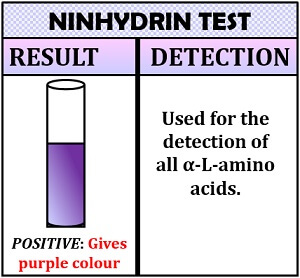
Inference: The appearance of purple colour indicates the presence of α -amino acids and yellow colour indicate imino acids’ presence.
Xanthoproteic Test
It is a specific test for detecting aromatic amino acids that contain active benzene ring or aromatic nucleus. The xanthoproteic test is based upon the principle of nitrification reaction. In this test, the active aromatic amino acids undergo nitrification in the presence of concentrated nitric acid and form a yellow coloured nitro-derivatives. The yellow colour turns into orange by the ionization of the phenolic group at alkaline pH.
Method:
- Prepare a 1 ml solution of the given sample.
- Then, add a few drops of nitric acid.
- Boil the solution for 2 minutes, and then cool the above solution.
Observation: Observe the tubes for the appearance of yellow colour.
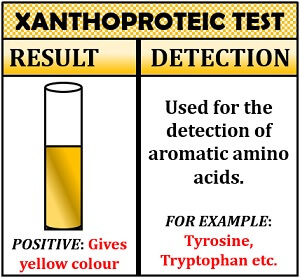
Inference: Appearance of yellow colour indicates the presence of aromatic amino acids.
Pauly’s Diazo Test
This test is specific for the detection of amino acids like histidine and tyrosine. The principle of Pauly’s diazo test is based upon the diazotation reaction. Pauly’s diazo test makes the use of chemical reagent (sulphanilic acid) which goes through diazotation reaction to form a diazonium salt in the presence of sodium nitrite and hydrochloric acid. The coupling of the diazonium salt with an amino acid (either tyrosine or histidine) gives a red colour to the alkaline medium’s solution.
Method:
- Take sulphanilic acid reagent in a test tube and place it inside an ice bucket to cool.
- Then, add prechilled sodium nitrite solution and few drops of chilled amino acid solution.
- At last, add sodium carbonate until the colour appears.
Observation: Observe the test tube for the appearance of red colour by adding sodium bicarbonate.
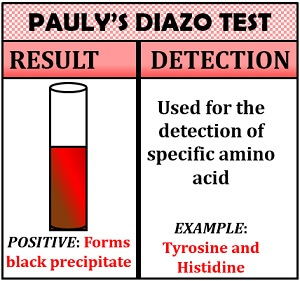
Inference: The appearance of the red colour will indicate the presence of tyrosine and histidine.
Millon’s Test
It is a specific test for the detection of phenolic amino acids like tyrosine. The principle of Millon’s test is based upon the nitration reaction. Millon’s test makes the use of a nitrifying agent, i.e. concentrated nitric acid, by which the phenolic amino acids are converted into nitrated amino acid by giving a red colour to the solution. The nitrated compound further forms a deeper yellow coloured salt of amino acids.
Method:
- First, take the sample of amino acid.
- Then, add Millon’s reagent to the above and mix all the contents.
- After that, boil the solution in a water bath for 5 minutes and then cool for a while.
- At last, add sodium nitrite solution until the colour appears.
Observation: Observe the test tube for the appearance of red colour.
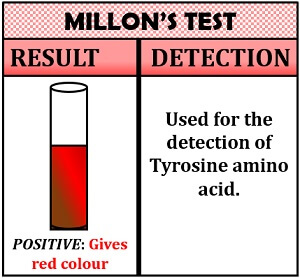
Inference: The appearance of a red colour indicates the presence of tyrosine.
Histidine Test
As from the name, it is clear that this test is specifically used to detect histidine’s presence. Knoop discovered this test. The principle of Histidine test is based upon the principle of bromination reaction. In Histidine test, bromination of amino acid occurs that gives a yellow coloured compound in the presence of bromine in acid solution.
Method:
- Prepare 1 ml sample of given amino acid.
- Then, add an acid solution containing 5% bromine in 33% acetic acid.
- After 10 minutes, add 2 ml of 5% sodium carbonate solution.
- Boil the solution in a water bath for 10 minutes.
Observation: Observe the test tube for the appearance of the blue colour in a solution.
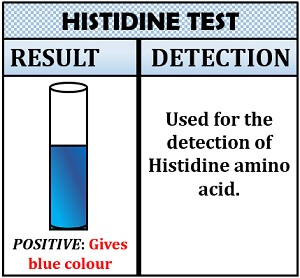
Inference: The appearance of a blue colour indicates the presence of histidine.
Hopkin’s Cole Test
This test is specific for the detection of tryptophan. The principle of Hopkin’s Cole test is based upon the dehydration reaction. In Hopkin’s Cole test, the amino acid reacts with the reagent glyoxylic acid in the presence of concentrated sulphuric acid. This reaction causes the dehydration of tryptophan, which forms a purple coloured ring between two solutions.
Method:
- Prepare 1 ml sample of given amino acid.
- Then add 1 ml of glyoxylic acid.
- Mix the contents and add concentrated sulphuric acid from the side of the tube in an inclined position.
Observation: Observe the test tube for the appearance of a violet ring in between the solution.
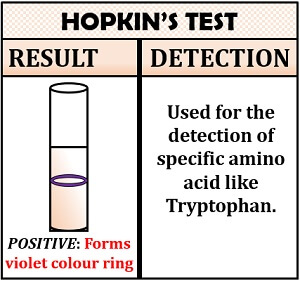
Inference: Appearance of a violet coloured ring will indicate the presence of tryptophan.
Sakaguchi Test
This test is specific for the detection of arginine. The Sakaguchi test is based on the principle of the oxidation reaction. Sakaguchi test makes the use of an oxidising agent, i.e. sodium hydroxide and α- naphthol reagent. Both sodium hydroxide and α- naphthol reacts with the arginine to give a characteristic red colour after hypochlorite treatment.
Method:
- Take 1 ml of given amino acid sample.
- Then, add 2 drops of sodium hydroxide.
- After that, add 2 drops of α- naphthol and mix all the contents.
- At last, add a few drops of hypochlorite solution.
Observation: Observe the test tube for the appearance of the red colour in the solution.
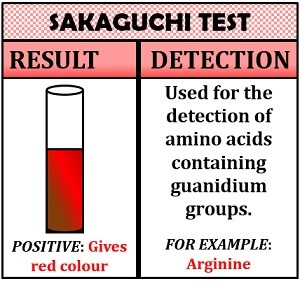
Inference: The appearance of the red colour indicates the presence of arginine.
Lead Sulphide Test
This test is useful for the detection of amino acids like cysteine, which contains the –SH or Sulfhydryl group. The principle of lead sulphide test is based upon the precipitation reaction. In the Lead sulphide test, the cysteine reacts with sodium hydroxide and converts into sodium sulphide after boiling all the contents. Then, sodium sulphide reacts with the lead acetate and goes through precipitation reaction by forming black precipitates as lead sulphide.
Method:
- Prepare 1 ml solution of given amino acid.
- Then add a few drops of 40% sodium hydroxide.
- Boil the solution in a water bath for about 5-10 minutes and then cool.
- At last, add 10% of lead acetate solution.
Observation: Observe the test tube for the formation of black precipitate in a solution.
Inference: Formation of a black precipitate indicates the presence of cysteine.
I cannot find complete information anywhere like this.
THANKS…………..
This article is very informative, thank you.