The qualitative analysis of carbohydrate is the significant test for detecting and classifying carbohydrates, depending on the colour change followed by chemical reactions. Carbohydrate is an organic biomolecule that has a chemical formula Cm (H2O) n. The carbohydrate consists of three atoms, viz. carbon, hydrogen and oxygen as the main constituent, where the ratio between the hydrogen and the oxygen is 2:1.
Carbohydrates act as the major energy source for all living organisms, and they are primarily of three kinds, namely mono, oligo and polysaccharides. Monosaccharides include simple crystalline sugars that are readily soluble in water. Oligosaccharides include low molecular weight sugars and exist as a polymer of monosaccharides covalently linked through glycosidic linkages.
Polysaccharides include high molecular weight sugars and exist either as a polymer of the same monosaccharides (homopolysaccharides) or different monomers (heteropolysaccharides). In this context, we will discuss the different chemical tests for the qualitative analysis of carbohydrates.
Content: Qualitative Analysis of Carbohydrate
- Meaning of Qualitative Analysis of Carbohydrate
- Video
- Methods for Qualitative Analysis of Carbohydrate
Meaning of Qualitative Analysis of Carbohydrate
The qualitative analysis of carbohydrates is detected based on the reagent’s utilisation and the reaction between the test sample and reagent. The reaction of the test sample with the chemical reagent gives a significant colour, through which we can detect the presence or absence of carbohydrates.
Video: Qualitative Analysis of Carbohydrate
Methods for Qualitative Analysis of Carbohydrate
We can determine the presence of carbohydrates in the test sample by employing the following chemical tests:
Molisch’s Test
It is the most common method for the detection of carbohydrates. Molisch’s test makes use of Molisch’s solution (contains α-naphthol in 95% alcohol) and concentrated H2SO4.
Principle: Molisch’s test detects the carbohydrate presence, which principle is based upon the dehydration reaction. The carbohydrates in the sample get dehydrated into aldehyde by the addition of concentrated H2SO4.
The aldehyde formed is either furfural (produced by the dehydration of pentoses or pentosans) or hydroxymethylfurfural (produced by the dehydration of hexoses or hexosans). Then, the α-naphthol in the molisch reagent reacts with the aldehyde and develops a purple condensation product.
Procedure:
- Take a sample and add water to make 2 ml of solution.
- Add 2 drops of Molisch’s reagent.
- Then, pour 5 ml of concentrated H2SO4 from the side of the inclined test tube.
- Observe the colour change in the tube.
Result interpretation:
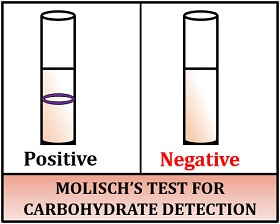
- Positive result: Violet ring appears at the junction of two layers, i.e. between the sugar and acid.
- Negative result: Green or brown colour appears.
Application: It detects the presence of all carbohydrates.
Benedict’s Test
It is also used for the analysis of carbohydrates as reducing sugars. Reducing sugar consists of a free aldehyde or ketone group. Benedict’s test makes use of Benedict’s solution as a chemical reagent, which contains copper sulphate, sodium citrate, and sodium carbonate with a pH of 10.5.
Principle: The reducing sugar will reduce into the enediols by reacting with an alkaline reagent, i.e. Benedict’s solution. The reducing sugar gives a green to brick-red colour precipitate depending upon the sugar concentration. The colour change is due to the reduction reaction of copper (II) to copper (I) in the solution that develops a red-coloured precipitate.
The resulting precipitate is water-insoluble. The sodium carbonate provides the alkaline condition or maintains the alkalinity in the redox reaction solution. Sodium citrate binds with the copper- II ions to prevent the reduction into copper I during storage.
Procedure:
- Prepare 1 ml of a solution by adding a test sample and water.
- Add 2 ml of Benedict’s solution to the test tube.
- Heat the solution in the water bath for 3 minutes.
- Observe the test tube for any colour changes.
Result interpretation:
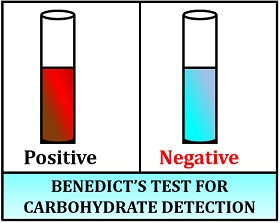
- Positive result: Green to brick-red precipitate forms.
- Negative result: Colour remains unchanged.
Application: It detects the presence of reducing carbohydrates.
Fehling’s Test
This method is also useful for the analysis of reducing sugars. It makes use of Fehling’s solutions A and B as chemical reagents. Fehling’s solution A contains copper sulphate pentahydrate in 1 L of distilled water. Fehling’s solution B contains potassium sodium tartrate and sodium hydroxide in 1 L of distilled water.
Principle: In Fehling’s test, a reduction reaction occurs between the aldehyde or keto groups of the reducing sugar and the alkaline cupric hydroxide that later reduces into cuprous oxide. This cuprous oxide gives a brick-red coloured precipitate in the solution.
Procedure:
- Add 2 ml of the test sample to a test tube.
- Then, add Fehling’s A and B solutions in equal proportion to the above sample.
- After that, place the test tube in a hot water bath for 3 minutes.
- At last, observe the colour change in a test tube.
Result interpretation:
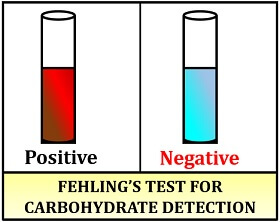
- Positive result: Brick-red precipitate forms.
- Negative result: Colour remains unchanged.
Application: It detects the presence of reducing carbohydrates.
Barfoed’s Test
This method is also used for the analysis of monosaccharide-reducing sugars. Barfoed’s test makes use of Barfoed’s solution, which contains copper acetate in the dilute acetic acid with a pH of 4.6.
Principle: In Barfoed’s test, the reducing monosaccharide is oxidized by the copper ion in the solution to form a carboxylic acid and copper (I) oxide, which results in the formation of a red-coloured precipitate.
Procedure:
- Add 1 ml of the test sample to a test tube.
- Then add 2 ml of Barfoed’s solution to the test tube.
- Place the tube in a water bath for boiling for up to 1 min.
- At last, observe the colour change in a test tube.
Result interpretation:
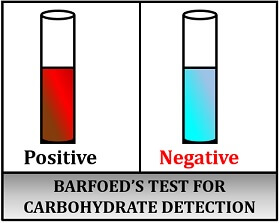
- Positive result: Gives red-coloured precipitate.
- Negative result: Colour remains unchanged.
Application: It determines the presence of reducing monosaccharides like glucose.
Bial’s Test
It is most commonly used for the detection of pentose sugars or pentoses. By this method, we can also differentiate pentoses from hexoses by means of colour change. Bial’s test makes use of Bial’s solution, which contains orcinol, hydrochloric acid and ferric chloride.
Principle: Here, the pentoses react with the Bial’s reagent and convert it into furfural derivatives due to dehydration by HCl. Then, orcinol and furfural condense in ferric ion presence and develop a green-coloured compound.
Procedure:
- Take 2 ml of the test solution in the test tube.
- Then, add 5 ml of Bial’s reagent.
- Heat the solution in a water bath for 1 min.
- Cool and observe the solution for any colour change.
Result interpretation:
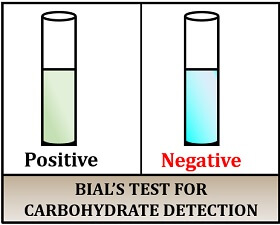
- Positive result: Gives green-coloured precipitate.
- Negative result: The solution remains unchanged.
Application: Used for the detection of the pentose monosaccharides like ribose.
Seliwanoff’s Test
It is most commonly used for the detection of Keto-sugars or Ketoses. By this method, we can also differentiate Ketoses from Aldoses by means of colour change. Seliwanoff’s test uses Seliwanoff’s reagent that contains 0.5 g of resorcinol per litre of 10% HCl.
Principle: In Seliwanoff’s test, ketoses react with the HCl of Seliwanoff’s reagent and yield furfural derivatives due to dehydration. Then, resorcinol and furfural react to give a deep red colour to the solution.
Procedure:
- Take 1 ml of test solution in the test tube.
- Then, add 3 ml of Seliwanoff’s reagent.
- Heat the solution in a water bath for 1 min.
- Cool and observe the solution for the colour change.
Result interpretation:
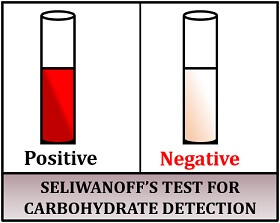
- Positive result: Gives a deep red colour to the solution.
- Negative result: Colour remains unchanged.
Application: Used for the detection of fructose, sucrose etc.
Iodine Test
It is widely used for the detection of starch in the solution. In an iodine test, iodine acts as an “Indicator”.
Principle: In the Iodine test, an iodine solution reacts with the starch (contains α-amylose and amylopectin polymers). This reaction between starch and iodine results in a starch-iodine complex, which gives a blue-black colour to the solution.
Procedure:
- Take 2 ml of the test solution in the test tube.
- Then, add 2 drops of iodine solution.
- Observe the solution for the colour change.
Result interpretation:
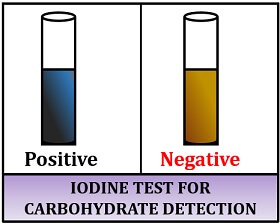
- Positive result: Gives blue-black colour to the solution.
- Negative result: Gives a brown-yellow colour to the solution.
Application: It detects polysaccharides like starch.