The spectrophotometer measures the absorption and spectral bandwidth of the given sample. Absorption is the logarithm of transmittance, i.e. Log (T), whereas transmittance is the portion of light moved through the sample.
Transmittance refers to the ratio of light incidents on the test sample to the light transmits through the solution, i.e. IO/I. Spectral bandwidth or bandpass refers to a range of colours that reflect through the test sample.
The spectrophotometer must be calibrated before taking the readings of the test sample by putting a cuvette holding a control solution. The process of calibrating the device commonly is called zeroing of the spectrophotometer.
The amount of light transmitted or absorbed can be calculated by the readings obtained in a digital meter of a spectrophotometer. This post discusses the definition, principle, types, components and applications of the applications.
Content: Spectrophotometer
Definition of Spectrophotometer
The spectrophotometer refers to an instrument that measures the absorbance of the test sample at a specific wavelength by measuring the amount of light transmitted by the sample. This device contains several components like a light source, collimator, monochromator, cuvette, light detector, and digital meter.
Spectrophotometry is a science which deals with the study of light intensity absorbed or transmitted by the different solutions once a ray of light moves through the test sample. Its basic theory concludes that each chemical compound transmits or absorbs certain light according to its relative wavelength.
Spectrophotometer Principle
The spectrophotometer principle depends upon the Beer-Lambert law, which states that a beam of light incidents on the homogenous solution, reflects some fraction of incident light, absorbs some light and transmits the remaining light through the solution.
Both Beer and Lambert have given their own theories on the absorption of radiation. According to Beer-Lambert law, the absorbance is equal to the given mathematical expression:
A= log I0/I
A= ε C l
Where, A= Absorbance of light
I0= Intensity of incident light
I= Intensity of transmitted light
ε= Absorption coefficient
C= Concentration of the absorbing material
l= Path length (cm)
The amount of light transmitted from the solution is inversely proportional to the absorption of light.
A= log10 1/T
As the absorbance of the medium increases, the transmittance of light through the solution decreases and vice versa. Absorbance is a non-dimensional quantity whose value ranges between 0-1.
Mechanism
A spectrophotometer includes the following sequential events:
- Firstly, a light source falls onto the monochromator (Dispersion device).
- Then, the monochromator will produce a single source of light that falls onto the focusing wavelength selector.
- The focusing convex lens will pass a fraction of the monochromatic light source from the sample solution to the photocell detector.
- A photocell detector converts the light energy into electrical energy, and an amplifier transmits this electrical signal to the internal circuit.
- Finally, an internal circuit inside a spectrophotometer gives out a final output on a digital meter.
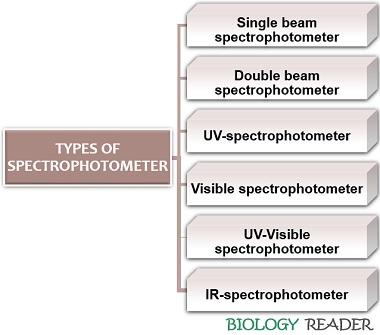
Types of Spectrophotometer
Based on Light Source
Single beam spectrophotometer
In this, a fraction of light from the diverging devices is wholly passed from the sample solution. A beam of light from the light source falls onto the collimator convex lens and moves to the diaphragm. The diaphragm ensures 100% transmittance and allows the light to fall onto the monochromator device.
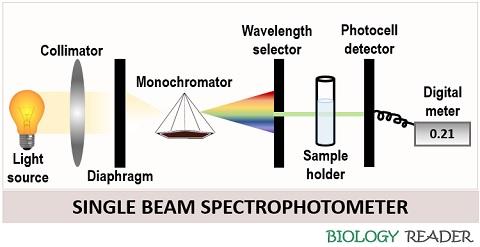
A dispersion medium or monochromator device allows the transmittance of a single source of light onto the focusing convex lens. The focusing convex lens transmits light of a particular wavelength from the sample to the photocell detector. A photocell detects the portion of light transmitted or absorbed and gives the reading on the display meter.
Double beam spectrophotometer
In this, a fraction of light coming from the monochromator device parts into two beams. One falls onto the reference sample and the other onto the test sample. Its mechanism is more or less similar to a single beam spectrophotometer but differs because the dual mirrors divide a single beam of light into two.
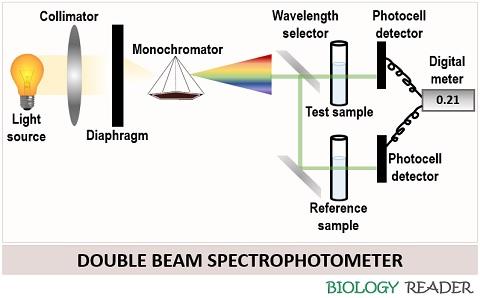
One beam of light passes from the test sample to the photocell, and the other passes from the reference sample to another photocell. A photocell detects the amount of light transmitted or absorbed and gives the reading on the display meter.
Based on Light Wavelength
Ultraviolet spectrophotometer
It uses cuvettes made of quartz and hydrogen or deuterium lamps as a light source. The hydrogen lamp emits continuous or discontinuous spectral UV- light ranging between 200-450 nm. This device determines the absorbance or transmittance for the fluids and even solutions.
Visible spectrophotometer
It uses plastic and glass cuvettes and a tungsten halogen light source. The tungsten lamp consists of a tungsten filament, emitting a visible spectral range between 330-900 nm. The tungsten lamp has a long life of 1200 h. This device can measure the change in colour intensity according to the change in the concentration of moderately diluted solutions.
Infrared spectrophotometer
It makes the use of Nernst glowers as a conductive device having a long life. This kind of spectrophotometer helps in studying the vibrations of different molecules at a specific wavelength. Near and mid-IR-rays cause rotational and harmonic vibrations.
Components of Spectrophotometer
A spectrophotometer includes the following elements:
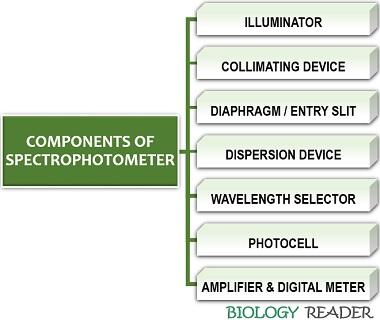
Illuminator
The illumination source usually includes hydrogen, tungsten, xenon flash lamps, and Nernst glower for the UV, Visible, UV-visible, and infrared spectrophotometer.
The light source emits continuous or discontinuous spectral bands at varying wavelength. An illuminator provides a polychromatic source of light to the collimating device.
Collimating Device
It is an optical device containing a tube with a convex lens on one side and an aperture on the other end. The collimator’s primary function to convert the radiating polychromatic light source into a parallel beam by the adjustable aperture. In the focal plane of a collimator convex lens, an opening or aperture passes the parallel beam onto the dispersion device via the diaphragm.
Dispersion Device
A dispersion device is positioned between the diaphragm and the wavelength selector. The diaphragm functions as an entry slit of the polychromatic source of light that ensures 100 % transmittance from the collimator to the dispersion medium.
The dispersion medium reflects the light of the selected wavelength through the exit slit, allowing the monochromatic light source to escape. Prisms, diffraction gratings and filter system are the most common dispersion medium.
Prism
It disperses the polychromatic source of light coming from the diaphragm into the constituent monochromatic source of light. A prism disperses the light of variable wavelength to a different extent, depending on:
- Its optical angle (60 degrees)
- And, the material from which it is composed
For commercial use generally, 60 degrees cornu quartz and 30 degrees littrow prisms are used.
Diffraction Grating
It refers to the optical components commonly used in the visible, UV and IR spectrophotometers. Its degree of dispersion depends upon:
- Spacing between the gratings
- Wavelength of light
Diffraction grating comprises of few ridges or rulings on the surface, coated by aluminium. It causes transmissive and reflective kind of diffraction.
Filter System
The filters are first layered with gelatin, then coloured with organic dyes and finally sealed within the glass plates. Now, these are the most common dispersion medium used for the biological and biochemical assays.
The filters can split the different parts of electromagnetic radiation by absorbing and transmitting a particular wavelength. Spectrophotometers consist of a filter system as a dispersion media and also called “Filter photometers”.
The optical filter provides a monochromatic source of light and contains absorption or interference filters. An absorption filter can transmit the desired range of wavelength or spectral elements by blocking some spectral components from a beam of light through an illuminator.
A filter wheel system is a commercial device incorporated with several filters having different wavelength responses and positioned nearly at the circumference of the rotating wheel. Each filter will show spectral bands at a particular wavelength. This kind of system only selects discrete bands.
Sample Holder
A cuvette is a sample holding tube that can be made of plastic, glass, fibre etc. A cuvette with a blank solution helps in calibrating the spectrophotometer by giving a zero reference number. The calibration of the spectrophotometer is necessary to check the accuracy of the light source.
Photocell
It refers to the photoresistor that detects the range of light transmitted from the test sample and transforms it into an electrical signal. A photocell detector shows the following properties:
- High sensitivity
- Short response time
- Long-term stability
- An electrical signal that can be easily amplified
Amplifier and Digital Meter
A photocell produces an electrical signal, which is directly proportional to the light reflected from the solution. An amplifier boosts the electrical signal and transfers it to the internal circuit, finally converting the signal into a readable form. The readings in a digital meter can be noted down, and a standard graph between absorption and transmittance can be plotted accordingly.
Applications
A spectrophotometer helps in the qualitative analysis of the properties like type, molecular weight and structure of different compounds, as the different compounds absorb light at different wavelengths. Aliphatic or acyclic hydrocarbons or their derivatives absorb light at a wavelength ranging between 220-280 nm.
It also helps in quantitative analysis of proteins, enzymes, amino acid (tyrosine), and blood glucose level by using a UV-visible spectrophotometer.