The active site of an enzyme is the region, which shows the highest metabolic activity by catalysing the enzyme-substrate complex into the products. The active site is found deep inside the enzyme, which resembles a hole or small depression. An active site is a region combining the specific substrate molecule with the enzyme and thus catalysing the reaction.
As we know the enzyme is “Highly specific” molecule, its specificity is due to the active site that allows the binding of a particular substrate. The amino acid residues are present around the active site, which holds the substrate molecule in the right position during biochemical reactions.
The substrate molecule shows a high binding affinity towards the active site. In this context, we will discuss the definition, reaction mechanism, characteristics, properties and role of the enzyme’s active site.
Content: Active Site of an Enzyme
- Definition of Enzyme’s Active Site
- Reaction Mechanism of an Enzyme
- Key Points
- Characteristics
- Role of Active Site
Definition of Enzyme’s Active Site
The enzyme’s active site is the small region, which seems like a cleft or cavity composed of nearly 10-15 amino acid residues. According to the term, we can define it as a site that activates the complex enzyme to bind with the particular substrate, induces the substrate’s transition state and stabilize the product formation. Thus, the active site merely refers to as the catalytic site. The active site performs two functional activities.
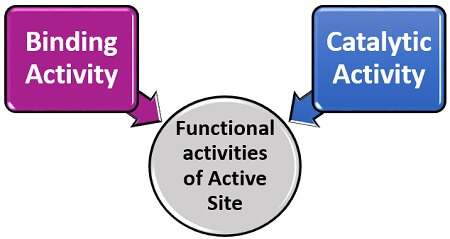 Binding Activity: The binding activity is a property of the enzyme’s active site, which increases the binding affinity of the substrate towards an enzyme.
Binding Activity: The binding activity is a property of the enzyme’s active site, which increases the binding affinity of the substrate towards an enzyme.- Catalytic activity: It is a property of an enzyme’s active site, which aids in the catabolic reaction of the enzyme and substrate to yield product by reducing the activation energy.
Reaction Mechanism of an Enzyme
In an enzyme-catalyzed reaction, the substrate will attach to the enzyme’s active site. A specific substrate will bind to the active site of an enzyme. As a result, an “Enzyme-substrate complex” forms. In the E-S complex, the substrate on enzyme activity will convert into a product. At last, the products get released, and the enzyme becomes free.
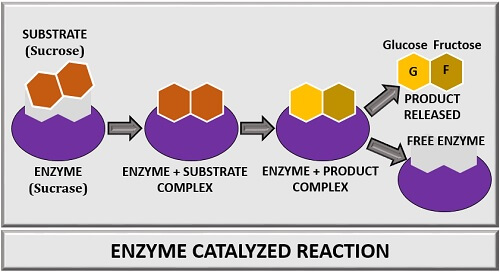
Example
Let us take an example, where sucrose is a substrate combining with the active site of an enzyme “Sucrase”. After, the binding of sucrose with an enzyme sucrase, an E-S complex forms. Then, a reaction between sucrase and sucrose takes place. The reaction will change the sucrose’s structural conformation called “Transition state of the Sucrose”. The change in sucrose’s structural configuration leads to the conversion of the E-S complex into the E-P complex. At last, glucose and fructose are released as products form the sucrase enzyme.
Key Points
- An active site is a specific location found in the enzyme where a substrate binds to catalyze the reaction. It is also called “Enzyme catalytic surface”.
- About 10-15 amino acid residues combine to form an active site.
- The active site possesses a specific geometrical shape and chemical signals that allow the specific recognition and binding between an enzyme and a substrate.
- An active site will allow the specific substrate to bind whose shape complements the shape of an active site. Therefore, a substrate is like is a key that can only fit into the particular lock, i.e. active site.
- The active site of an enzyme catalyzes many chemical or biological pathways.
- After the enzyme-substrate complex formation, both substrate and active site change its structural configuration by bending the target bonds and breaking the substrate molecule into a product.
- Enzymes show catalytic activity, which is due to its active site. It catalyzes a substrate into a product after complementary binding of the substrate with the active site of an enzyme, depending upon the geometric shape, size, charge and stereospecificity etc.
- The active site of an enzyme induces the “Transition of the substrate”.
Important Characteristics
Following are the important characteristics of an active site that includes:
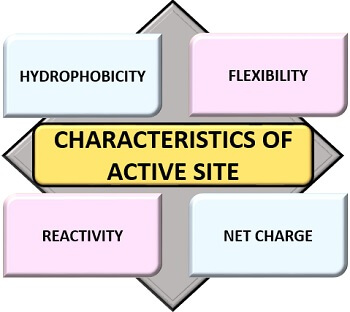
Hydrophobicity
The initial binding of substrate and enzyme occur through the non-covalent bond. But, the catalytic site involves hydrophobic interaction in the attachment of a substrate with an enzyme. Hydrophobic binding of the substrate to the active site of an enzyme increases the binding affinity. Other than hydrophobic interaction, there are three other mechanisms, like Vander Waal, hydrogen bond and electrostatic force of interaction, which also promotes E-S complex formation.
Flexibility
An active site shows flexibility as it can change its conformation to mediate substrates’ conversion into products.
Reactivity
The active site of an enzyme reacts with the specific substrate. Its reactivity depends on the environmental conditions like temperature, pH, enzyme and substrate concentration, etc. The enzyme’s active site combines with the substrate and thereby reduces the activation energy to further catalyze the reaction.
Net charge
The active site mainly consists of non-polar amino acid residues, which carry no charge or zero net charge. Some active site also consists of polar amino acids, which carry both positive and negative charge. The net charge of the catalytic site decides which amino acid will bind with the enzyme. There must be a complementary pairing between the active site and the substrate. The same charge on both the catalytic site and substrate will not form an E-S complex as repulsion may result between the two.
Role of Active Site
As we have discussed the active site performs two major activities like:
- The binding of a substrate with an enzyme.
- The catalytic activity by converting substrate into product.
Let us assume two conditions; one is the conversion of substrate into a product without enzyme and second in an enzyme’s presence.
First condition
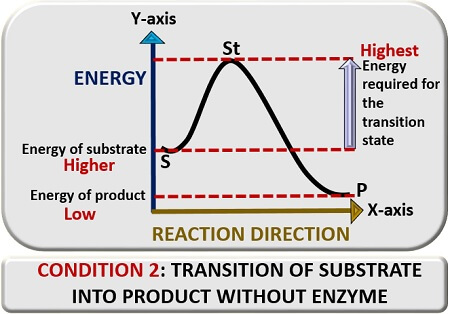
In the first condition, we will discuss the substrate’s transition reaction into a product in the absence of an enzyme catalyst. For this, plot a graph between reaction direction and energy. In the absence of a “Catalyst”, a substrate (S) will require higher “Activation energy” to go into the transition state, which we will represent as “St”. In the transition state, the substrate will change its conformation and thereby release a product (P).
Second condition
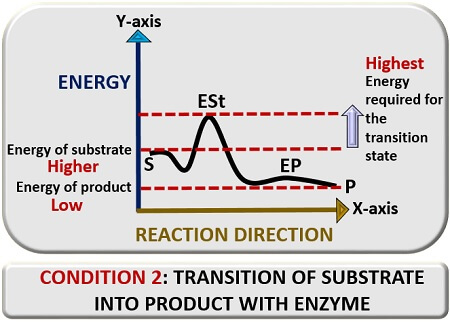
Here, we will discuss the transition reaction of the substrate into a product in the presence of an enzyme catalyst. For this also, plot a graph between reaction direction and energy. In the presence of a “Catalyst”, a substrate (S) will bind to an enzyme’s catalytic site. The enzyme is a catalytic agent, which will mediate catalysis of a substrate.
The enzyme will modify the substrate and take it to the transition state, which we will represent as “ESt”. In the transition state, the enzyme and substrate will react, and there a change occurs in the substrate’s configuration. This change will lead to the formation of the enzyme product complex and finally release a product (P).
Therefore, we can conclude that an enzyme’s active site lowers the activation energy by increasing the rate of reaction. The substrate’s energy level is higher than that of a product but lower than the transition state of the substrate. The activation energy is inversely proportional to the reaction rate, i.e. it decreases with the increase in the rate of reaction.