Animal cell culture is one of the important tools now in the field of life science. It is the in-vitro technique, in which the cells are grown in the laboratory conditions under proper nutrient source, growth factors and the controlled environmental conditions for the cell growth and division.
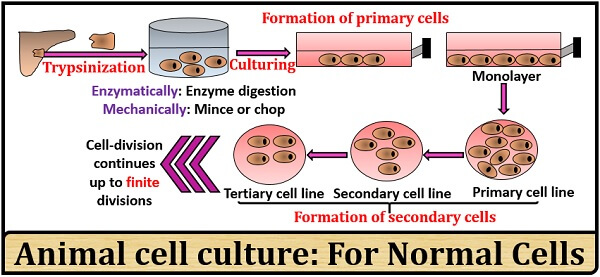
The cells in the animal cell culture are obtained by either enzymatic action like trypsinization or mechanically by mincing or chopping. In animal cell culture, the cells form a monolayer in the solid media and suspension in the liquid media. The method of a cell or tissue culture first involves the production of primary cells and followed by subculturing or passaging, it can produce secondary cells lines.
In this context, we will discuss the contributions that have led to the discovery of animal cell culture along with the definition, types, process, advantages and limitations. Also, you will get to know the process of animal cell culture in the normal cells as well as continuous cells.
Content: Animal Cell Culture
- History of Animal cell culture
- Definition of Animal cell culture
- Types of Animal cell culture
- Process of Animal cell culture
- Difference between Normal and Continuous cells
- Advantages
- Disadvantages
- Applications
History of Animal Cell Culture
There are many discoveries which led to the invention of the technique named animal cell culture. By the discovery of animal cell culture, there are some other discoveries too, which made the practical study of cell culture possible.
Firstly the antibiotics were developed to prevent contamination. Secondly, the cell obtaining techniques were introduced to produce cell lines by trypsin. Then, standard media were introduced, which made the growth of cells far easier and faster.
| Year | Discoverer | Discovery |
|---|---|---|
| 1878 | Claude Bernard | Discovered mechanisms to maintain the living state of the organism after its death |
| 1885 | Raux | Discovered the maintenance of the embryonic chick cells in the saline culture |
| 1897 | Loeb | Discovered the survival of the cell isolated from blood and connective tissue in the serum and plasma |
| 1903 | Jolly | Demonstrated the cell division of Salamander leucocytes by an in-vitro method. |
| 1907 | Harrison | Discovered the growth of nerve fibres by cultivating the frog nerve cell in a lymph clot by hanging drop method |
| 1910 | Burrows | Introduced the cultivation of chicken embryo-cell in the plasma clots |
| 1911 | Lewis | Introduced a liquid medium containing sea water, serum, embryo extract, salts and peptones |
| 1913 | Carrel | Introduced certain aseptic techniques for the long term culturing of cells |
| 1916 | Rous and Jones | Introduced trypsin (Proteolytic enzyme) for the subculturing of confluent cells |
| 1923 | Carrel and Baker | Developed “Carrel” or “T-shaped” culture vessel for the cell |
| 1927 | Carrel and Rivera | Introduced first viral vaccine “Vaccinia” |
| 1933 | Gey | Introduced Roller tube technique |
| 1940 | Introduction of antibiotics like penicillin and streptomycin in cell culture to avoid contamination | |
| 1948 | Earle | Isolated L-fibroblasts from the clones of single cells |
| 1948 | Fischer | Introduced chemically defined medium CMRL 1066 |
| 1949 | Enders | Reported the growth of polio virus on the human embryonic cells |
| 1952 | Gey | Discovered the growth of continuous cell line from Hela- cells |
| 1952 | Dulbecco | Discovered plaque assay technique for animal viruses using confluent monolayers of the cultured cells |
| 1954 | Abercrombie | Introduced the property of “Contract inhibition” |
| 1955 | Eagle | Introduced chemically defined medium for the cell culture |
| 1961 | Hay Flick and Moorhead | Showed finite cell culturing by isolating human fibroblast WI-38 |
| 1964 | Littlefield | Introduced HAT-medium for cell selection |
| 1965 | Ham | Introduced serum free medium |
| 1975 | Kohler and Milstein | Produced hybridoma capable of producing monoclonal antibody |
| 1978 | Sato | Established the basis for development of serum free media |
| 1982 | Introduction of human insulin as a therapeutic agent | |
| 1986 | Lymphoblastoidy γIFN has been licensed | |
| 1987 | Introduction of Tpac (tissue type plasminogen activator) | |
| 1989 | Introduction of erythropoietin | |
| 1990 | Introduction of recombinant products (HBsAG, factor VIII, HIV gp 120, CD4) |
Definition of Animal Cell Culture
Animal cell culture is defined as the type of cell culture, in which the cell grows and multiplies either in a solid or liquid medium as a cell monolayer or cell suspension, respectively to produce the primary cells. Then, followed by subculturing of primary cells, it produces secondary cells to a definite cell number in the normal cells and indefinite in the continuous cells.
Types of Animal Cell Culture
On the basis of cell growth and division, the animal cell culture is subdivided into the following two types:
- Primary cell culture
- Secondary cell culture
Primary Cell Culture
It is defined as the cell culturing from the tissue of the host animal. The cells can be obtained directly by the mechanical method and indirectly by the enzymatic action. Once, the cells are obtained, they have to be cultured on a suitable container that must be provided with all the nutrients required for the cell division and growth. The growth of primary cells either occurs as an adherent monolayer on the solid media or as a suspension in a liquid medium.
- Adherent cells: These cells adhere to the solid surface and produce a cell population in the monolayer pattern. Adherent cells are sometimes called a confluent cell, in which the cells merge or contract to fill the surface area. Fibroblasts and epithelial cells are examples of adherent cells. The properties of the adherent cells include:
- Adherent cells are anchorage-dependent.
- Grows in a monolayer pattern.
- Growth occurs on the solid surface or we can say on the solid media.
- Adherent cells fill the entire surface area of the container or vessel as a monolayer.
- Adherent cells follow the property of contract inhibition, in which the cell itself ceases the growth of some cells to maintain the synchronized state through chemical signalling. Once a monolayer is formed, the formation of new cells is inhibited by the adherent cells.
- Suspension cells: These are the cells that do not adhere to the surface of the medium. Suspension cells are the type of cells that float as the suspension over the liquid medium. The properties of the suspension cells include:
- Suspension cells are anchorage-independent.
- Floats as a suspension of cells over the liquid culture medium.
- Growth occurs in the liquid nutrient medium.
- Suspension cells grow much faster than the adherent cells.
- There is a short lag phase in the suspension cells.
- Enzyme action is not required for the dissociation of cells.
Secondary Cell Culture
It is defined as the subculturing of the primary cells, which later produce secondary cells lines. The passaging or subculturing of the primary cells result in a phenotypic and genotypic uniformity of the cell population. After the subculturing, the cell-lines will become different from the original cells. Based on the cell’s life span, the cell lines can be categorized into:
- Finite cell lines: Here, the cells possess a limited life span and show a limited cell division. Passaging value is less because the cells after some time lose the ability to grow or proliferate and enter into the phase of senescence or ageing.
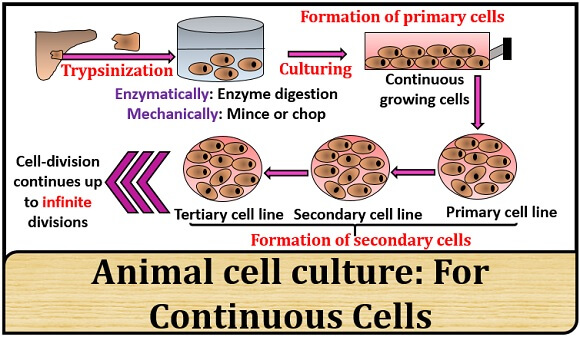
Example: Normal cells produce finite cell lines. - Continuous cell lines: In continuous cell lines, the number of cell division and passaging value is indefinite. The passaging value is more because the continuous cells do not lose the ability to divide, i.e. these can grow and divide by an infinite number of times.
Example: Cancerous cells produce infinite or continuous cell lines.
Process
The process of animal cell culture can be summarized into the following series:
- Tissue explant: It involves the removal of tissue from the organ.
- Cell extraction: It is carried out either mechanically or enzymatically. The extraction is mostly carried out by the enzyme action or by the process of trypsinization.
- Culturing in a nutrient medium: After that, the cells are cultured either on a solid nutrient medium or liquid nutrient medium. The primary cells form a monolayer over a solid nutrient medium, whereas appears as a cell suspension over the liquid medium.
- Subculturing: It is also called cell passaging. The subculturing is carried out after the formation of primary cells and it is important to continuously study or to grow the cells. This is the most important step in cell culture, which helps us to understand the cell type.
Normal cells: These cells possess a low passaging value because they lose their ability to divide after some time due to cell ageing. Therefore, the cell divides to produce definite cell lines.
Continuous cells: These cells possess a high passaging value because the cells have the ability to continuously divide. Therefore, the cell divides to produce indefinite cell lines.
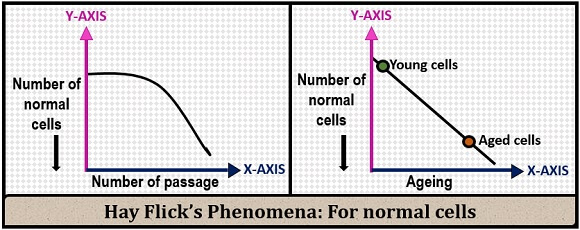
Passaging value is directly proportional to the cell type and cell division.
- For continuous cells, the passaging value increases, by which the cell division will be more.
- For normal cells, the passaging value decreases as the cell ages, by which the cell division capacity will also decrease.
Difference Between Normal and Continuous Cells
- The normal cell produces a monolayer, whereas the continuous cell does not.
- The continuous cell does not follow the property of contract inhibition, while the normal cells follow.
- A normal cell has a property to divide for definite times, whereas the continuous cell has a property to divide again and again.
- The continuous cells possess a high passage value, whereas the normal cells possess a low passage value.
- The capacity of dividing in the continuous cell remains the same as the first passaging, whereas the capacity of dividing decreases as a result of cell ageing in the normal cells.
Advantages
- Animal cell culture makes the use of a low amount of reagents.
- Serial passaging maintains the homogeneity of the cell types.
- Provides controlled physiological conditions.
- Provides controlled physiochemical conditions like temperature, oxygen concentration, ph etc.
Disadvantages
- Animal cell culture requires high technical skills to interpret and to regulate the animal cell culturing.
- It is a very expensive method to carry out.
Applications
The animal cell culture provides a model to study the effects of drugs, biochemistry etc. of the cell. By animal cell culture, we can perform tissue engineering. It also helps us to identify the cancerous cell as both the normal and continuous cells can be grown in the animal cell culture.
We can also study the replication and life cycle of the virus, so it plays an important role in the field of virology. In animal cell culture, we can also study the effect of different drugs on different cell types, so it also helps in toxicity testing.