Chemical disinfectants either destroy, inactivate or inhibit the pathogenic growth. Disinfection eliminates various microbes, except for bacterial spores, using different chemical agents, heat or radiation. It disinfects the inanimate objects. Disinfection is classified into low-level, intermediate-level or high-level.
Low-level disinfection is efficient in killing vegetative bacteria and lipid-containing viruses. Intermediate-level disinfection effectively kills bacteria, fungi and viruses. High-level disinfection is a process of killing all microbial pathogens, except bacterial endospores.
Unlike sterilization, disinfection needs shorter exposure. It is less effective than sterilization that kills all the life forms, including bacterial and fungal spores. This post describes the definition of chemical disinfection and the different types of disinfectants used in chemical disinfection.
Content: Chemical Disinfectants
- Disinfection Meaning
- Disinfectants Meaning
- Factors Affecting
- Ideal Properties
- Types of Chemical Disinfectants
- Conclusion
Disinfection Meaning
Disinfection merely refers to the process that involves the addition of chemical disinfectants to the surface of inanimate objects, which in turn react with the structural components of microbial organisms.
Disinfectants Meaning
Disinfectant is a chemical substance that destroys or inactivates various life forms (bacteria, fungi and viruses) on inert surfaces. Sometimes, it is interchangeable with the terms mentioned below, but the mode of action differs in a certain way:
- Antibiotic: Different from an antibiotic that works within the body.
- Antiseptic: Different from antiseptic that destroys microorganisms on living tissues.
- Biocides: Different from biocides that kill all life forms, not just microorganisms.
Classification: Liquid and gaseous disinfectants are the two common forms of disinfectants based on consistency. Based on the spectrum, disinfectants are high, medium and low levels. Disinfectants show different modes of action.
- Disrupts the cell membrane
- Coagulates cellular proteins
- Oxidize essential sulfhydryl groups of enzymes
- Interferes with the cell metabolism
- Damages nucleic acids
Factors Affecting Potency of Chemical Disinfectants
Temperature, pH, nature of the organisms, the presence of other chemicals, disinfectant concentration, stability and storage of disinfectants etc., are the factors that may affect the potency of the disinfectants.
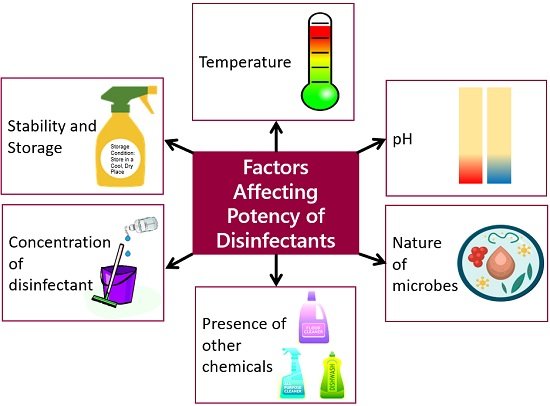
Temperature
In general, most disinfectants work best at temperatures above 68 degrees Fahrenheit. The disinfectant may evaporate at a temperature above 68 degrees Fahrenheit and colder temperatures may also reduce the efficacy of the disinfectant as well.
pH
It also affects the potency of the disinfectants. For example, glutaraldehyde works best at a pH above seven, and quaternary ammonium compounds are effective at a pH of 9-10. The pH can also affect the activity of phenol, chlorine and iodine compounds.
Nature of the organisms
The efficacy of disinfectants varies with the nature and type of organisms. Based on the different groups of microorganisms, disinfectants are classified into high, intermediate and low levels. Some kill microbes like bacteria and fungi, while some can effectively kill vegetative cells, enveloped viruses and mycobacteria.
Presence of other chemicals
They can affect the efficacy of some disinfectants. For example, the quaternary ammonium compounds may affect the effectiveness of iodine compounds, while phenols with soaps increase the penetrative ability.
Disinfectant concentration
The effective concentration of disinfectant is necessary to achieve successful disinfection. The dilution of a disinfectant differs depending upon the microbiostatic action and microbiocidal action. Microbicidal is an activity of killing microorganisms, and microbiostatic action refers to the activity of inhibiting microbial growth. Over dilution may render the efficacy of disinfectants. Thus, proper dilution is necessary for the best results, for which you can refer to the product label.
Stability and Storage
Disinfectants (like sodium hypochlorite) lose stability when stored over long periods, especially in the presence of heat or light. The product label list mentions the shelf life of the concentrated disinfectant. Disinfectants are generally kept in a dark and cool place.
Ideal Properties of Disinfectants
Ideal disinfectants should possess the following properties:
- They should have a broad spectrum of microbial activity.
- High penetrating power and stability.
- It should be effective in acid as well as alkaline media.
- Should be active in the presence of organic matter.
Unfortunately, disinfectants are not ideal. Therefore, to select the most effective and cost-efficient disinfectants, some careful considerations are mentioned below.
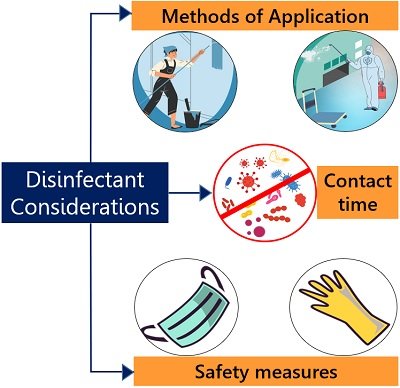
Contact Period
It is a given period of time for a type of disinfectant to destroy or inhibit the microbial activity at a given concentration. It is the minimal time stated by the manufacturer on the product label. In simple words, the contact time refers to the time limit for the chemical necessary to be left on the surface to kill the desired bacteria. For example, alcohol-based sanitizers have a contact time ranging between 30 seconds and 15 minutes to kill pathogenic microbes, whereas 3% phenol requires 2-3 hours.
Methods of Application
Disinfectants can be applied in several ways. A disinfectant solution can be used to disinfect floors, walls and other objects by wiping and brushing. A disinfectant spray acts as a fumigant that maintains the aseptic condition of rooms.
Safety Measures
Most disinfectants are irritant or potent sometimes to eyes, ears, mouth, irritated skin and other sensitive parts. Therefore, one should consider the safety of all personnel. Before using the given disinfectant, a person must read the product label or instructions about the active ingredients, direction for use, storage condition, caution, expiry and other details. Some disinfectants should be handled with care and you may use gloves, masks and eye protection.
Types of Chemical Disinfectants
The chemical disinfection method uses various chemical disinfectants that we have discussed below:

Alcohols
- Reagents: Ethyl alcohol, isopropyl alcohol and methyl alcohol.
- Mode of action: They coagulates bacterial proteins, disrupt membranes by solubilizing lipids and dehydrate cells.
- Effective against: Concentration of 60-90 % of alcohols is potent for lipid-containing viruses and non-sporing bacteria.
- Applications: Ethanol can be used as a skin antiseptic, isopropyl alcohol disinfects instruments and methanol is extensively used to wipe down the interior of biological safety cabinets.
- Disadvantages: Alcohol compounds are generally non-corrosive, inflammable and volatile.
Aldehydes
- Reagents: Formaldehyde, paraformaldehyde and glutaraldehyde.
- Mode of action: Alkylates protein groups (amino-, carboxyl- or hydroxyl group) and damages nucleic acids.
- Effective against: All microorganisms, including spores.
- Applications: 40% Formaldehyde (formalin) helps in surface disinfection. The concentration of 7.0-73% v/v of paraformaldehyde in the air decontaminates rooms, chambers, biological safety cabinets. 10% formalin with 0.5% tetraborate sterilizes clean metal instruments. 2% of glutaraldehyde is effective in sterilizing equipment like thermometers, centrifuges etc.
- Disadvantages: Fumes of aldehydes are irritating due to pungent odour. They have poor penetration and sometimes behave as carcinogens.
Halogens
- Reagents: Chlorine compounds (like chlorine, bleach etc.) and iodine compounds (like iodophores and tincture iodine).
- Mode of action: Oxidation of essential sulfhydryl groups of enzymes. Chlorine reacts with water to form hypochlorous acid, which is microbicidal.
- Effective against: It shows bactericidal and virusidal activity.
- Applications: Tincture of iodine serves as an antiseptic. 10% undiluted povidone Iodine (an iodophore) is useful in pre and postoperative skin disinfection. Chlorine gas is used to bleach water. Household bleach disinfects floors. 0.5% of sodium hypochloride is used in serological tests to study the nature of viruses.
- Disadvantages: Iodine and chlorine are corrosive.
Heavy Metals
- Reagents: Mercuric chloride, silver nitrate, copper sulfate, organic mercury salts etc.
- Mode of action: They cause precipitation of proteins and oxidation of sulfhydryl groups.
- Effective against: They are bacteriostatic.
- Applications: Silver sulphadiazine prevents colonization and infection of burn tissues. Merthiolate at a concentration of 1:10000 preserves serum. Copper salts act as fungicides.
- Disadvantage: Mercuric chloride is highly toxic.
Dyes
- Reagents: Aniline dyes (brilliant green, malachite green etc.) and acridine dyes (like proflavine, acriflavine etc.). Aniline dyes are non-irritant and non-toxic to the living tissues. Organic matter minimally affects the acridine dyes.
- Mode of action: They are bacteriostatic at high concentrations. Aniline dyes react with the acid groups in the bacterial cell. Acridine dyes impair the DNA complexes of microorganisms and prevent replication.
- Effective against: They are more active against gram-positive bacteria.
- Applications: They are used as a skin and wound antiseptics.
Phenols
- Reagents: Carbolic acid, Lysol, cresol etc.
- Mode of action: They disrupt cell membranes, cause precipitation of proteins and inactivate enzymes.
- Effective against: They are bactericidal, fungicidal and mycobactericidal.
- Applications: Carbolic acid, Lysol and cresol are the common disinfectants that disinfect interiors of hospitals, rooms etc. Chlorhexidine is a skin antiseptic used in wound dressing.
- Disadvantages: Skin irritant, toxic and corrosive.
Surface Active Agents
- Reagents: Soaps and detergents.
- Mode of action: They act on the phosphate groups that disrupt the cell membrane and denature cell proteins of microorganisms.
- Effective against: Surface active agents with a dilution of 1-2% is efficient to destroy vegetative cells, Mycobacteria and enveloped viruses.
- Applications: Surface-active agents can be used as disinfectants, detergents and emulsifiers.
- Disadvantages: Anionic detergents and organic matter reduce the efficacy of surface-active agents.
Conclusion
Therefore, disinfectants are the chemical agents disinfecting inanimate objects. To maintain the efficacy of disinfectants, we must know the required effective dilution, the contact time of disinfectant, applying procedure and storage conditions.