The difference between agarose and polyacrylamide gel relies upon factors like origin and molecular complexity. Both gels help in biomolecular separation, due to their porous nature. We can separate, identify and purify different biomolecules using agarose and polyacrylamide gels.
1. Origin: Agarose is a natural polymer extracted from several red seaweeds. So, it is a marine-based polysaccharide. But, polyacrylamide is a synthetic polymer. Free-radical polymerization of acrylamide and bisacrylamide produces polyacrylamide.
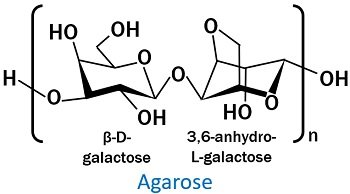
2. Molecular complexity: Agarose is a complex polysaccharide. It exists as a linear polymer with repeating units of agarobiose.
- β-D-galactose
- 3,6-anhydro-L-galactopyranose
The above two constitute the structure of a disaccharide ‘Agarobiose’.
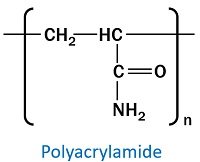
In contrast, polyacrylamide has a simple, repeating, linear chain structure with acrylamide subunits. But, the linear structure converts into cross-linked polymer via the addition of bis-acrylamide.
- Acrylamide is an organic compound having the chemical formula CH2=CHC(O)NH2.
- Bis-acrylamide acts as a crosslinker and creates crosslinks between acrylamide monomers.
3. Electrophoretic separation: Polyacrylamide gel is good for isolating small biomolecules like:
- Majority of proteins
- Small DNA fragments (5-500 bps).
In contrast, an agarose gel matrix is applicable for separating large biomolecules like:
- Protein complexes with molecular density >200 kDa.
- DNA fragments (50-20,000 bps).
This post describes the key differences between agarose and polyacrylamide gel. Also, we will discuss the definition, properties, uses, pros and cons between the two.
Content: Agarose Vs Polyacrylamide Gel
Comparison Chart
| Properties | Agarose Gel | Polyacrylamide Gel |
|---|---|---|
| Meaning | It is a polymer made of agarobiose monomers | It is a polymer composed of acrylamide molecules |
| Type | Natural polymer | Synthetic polymer |
| Molecular formula | C24H38O19 | (C3H5NO)n |
| Chemical bond | Hydrogen bond holds the agarose molecules | Covalent cross-linking holds the acrylamide molecules |
| Nature of gel | Less stable | Chemically more stable |
| Extraction | It is extracted from agar | It is extracted from acrylic acid |
| Toxicity | Non-toxic | Potent neurotoxic |
| Molecular complexity | Complex | Comparatively simple |
| Gelling property | It gels into a 3-D meshwork | It gels into a molecular sieve |
| Settling time | Low settling time | Takes more time to settle |
| Cost | Expensive | Less expensive |
| Operation in electrophoresis | Easy and simple | Time consuming and tedious |
| Concentration range for electrophoresis | 0.5-2% | 6-15% |
| Resolving power | Low | High |
| Run configuration | Horizontally run | Vertically run |
| Gel casting | Agarose gel sets as it cools | It sets by a chemical reaction once cross-linking occurs |
| Pore size | Large and non-uniform | Small and uniform |
| Application | Good for separating large DNA molecules | Good for separating small proteins and DNA fragments |
Definition of Agarose Gel
It is a polysaccharide containing repeating units of agarobiose. Intermolecular forces hold the alternating agarose molecules together. Agarose is a natural polysaccharide. Through red algae (Gelidium and Gracilaria), the extraction of agarose is possible.
Properties
- Agarose is the principal component of a complex polymer, i.e. agar. Extraction of agarose is through the separation of agaropectin (protein component) from agar.
- It has a molecular weight of 120,000 Da.
- Physical state: White powder
- Agarose is an organic compound that exhibits “Thermal Hysteresis” or “liquid-gel transition”. At a specific temperature range, agarose transforms into a gel or liquid.
- Gel: At temperature between 34-52 degrees Celsius, agarose gels. The helical fibre bundles of agarose form a 3D mesh of channels at this state. Hydrogen bond holds each bundle together.
- Liquid: Agarose gel melts near-boiling water temperature (85-95 degrees Celsius). Here, the helical fibres combine to form a supercoiled structure. Thus, agarose has a random coil structure during this state.
- Melting and gelling temperature depends upon agarose concentration.
- Low agarose concentration=High gel strength
- High agarose concentration=Low gel strength
Gel strength is the force applied to depress a surface of a gel.
- Low agarose concentration= Large pore size of the gel matrix.
- Thus, 0.5 to 2% of agarose is preferable to perform electrophoresis. Due to less concentration, large pores in the gel matrix separate large biomolecules. Thus, agarose gel electrophoresis is best to separate large protein complexes and DNA molecules.
- Agarose gel has low resolving power.

Applications
The gelling property of agarose makes it suitable to perform many applications. It provides a support matrix to culture, isolate and purify different biomolecules. Also, it helps to perform different bioassays.
- Preparation of cell culture media
- Electrophoretic separation of nucleic acids
- Immunodiffusion and immunoelectrophoretic techniques
- Purification of proteins
- Motility assays
The table given below displays the pros and cons of the agarose gel:
| Pros | Cons |
|---|---|
| Agarose gels are non-toxic and easy to handle | High cost of agarose |
| Gels set quickly and casting is easy | Fuzzy bands |
| Good for separating large DNA molecules | Poor separation of low molecular weight samples |
| Sample recovery is easy by melting the gel | Pore size is non-uniform |
| Different buffers can be used to alter the resolution | Low percentage gels may break when lifted |
| Pore sizes can be regulated by increasing or decreasing molecular sieving | High percentage gels may not set evenly |
Definition of Polyacrylamide Gel
It is a synthetic polymer containing repeating acrylamide subunits. Covalent cross-linking holds the chain of acrylamide molecules. It is a polyolefin (a synthetic polymer produced via olefins polymerization).
Properties
- Acrylamide (C3H5NO) is an outcome of the hydration of acrylonitrile via nitrile hydratase.
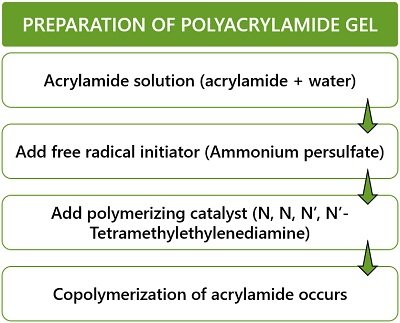
- Acrylamide is available as a powdered form. Its polymerization occurs via a free radical reaction. The reaction requires:
- Free radical initiators (like ammonium persulfate).
- N, N, N’, N’-Tetramethylethylenediamine (TEMED) as a polymerizing catalyst.
- Mixing of acrylamide solution with free-radical initiator initiates polymerization. As a result of this reaction, a linear polyacrylamide chain forms.
- By introducing cross-linkers to the above reaction, crosslinking occurs. Bis-acrylamide is a common cross-linking agent forming a highly branched chain of acrylamide.
- By preparing polyacrylamide gels, the pore size can be regulated. The porosity of the gel matrix depends upon the ratio of acrylamide to bis-acrylamide.
- High acrylamide concentration=Small pore size (Resolves smaller proteins better).
- Low acrylamide concentration=Large pore size (Resolves larger biomolecules).
- Polyacrylamide gels are polydisperse in structure and serve as a molecular sieve.
- To perform polyacrylamide gel electrophoresis (PAGE), 5-25% concentration of acrylamide is preferable.
Applications
Polyacrylamide gel is a non-ionic, water-soluble, and biocompatible polymer. Thus, it can be tailored to meet a broad spectrum of applications.
- Pulp and paper production
- Food processing
- Mining
- Flocculant in the wastewater treatment
- Oil extraction and recovery
The table given below interprets the pros and cons of the polyacrylamide gel:
| Pros | Cons |
|---|---|
| Chemically stable cross-linked gel | Requires new gel preparation after each experiment |
| Gives reproducible results by producing sharp bands | Gels are tedious to prepare |
| Good for separating biomolecules of small size | Toxic monomers |
| Pore size is uniform | Care must be taken while handling solutions of acrylamide |
| It has no charge. Hence, it is non-reactive with samples | Gel leakage often occurs |
| Thin gel casts promote better separations | |
| Very high resolving power |
Key Differences Between Agarose and Polyacrylamide Gel
- Agarose (C24H38O19) is a natural polymer made of agarobiose monomers. Whereas, polyacrylamide (C3H5NO)n is a synthetic polymer composed of acrylamide molecules.
- The hydrogen bond holds the agarose molecules. Thus, the nature of gel is less-stable. In contrast, covalent cross-linking holds the acrylamide molecules. Thus, polyacrylamide gel is chemically more stable.
- We could extract agarose by excluding a protein component (agaropectin) from agar. While polyacrylamide contains monomers of acrylamide. And, the hydration of acrylonitrile via nitrile hydratase produces acrylamide.
- Agarose is non-toxic. But, acrylamide is a potent neuro-toxic.
- Gelation of agarose requires less time and produces a 3-D meshwork. Whereas, polyacrylamide gels into a molecular sieve and takes more time to settle.
- The operation of AGE is easy and simple, but the resolving power is low. In contrast, PAGE is time-consuming and tedious. Yet, the resolving power is high.
- In AGE, the running configuration of agarose gel is horizontal. Although, the running configuration of polyacrylamide in PAGE is vertical.
- The pore size of agarose gel is somewhat larger and non-uniform. Whereas, polyacrylamide gels have a small and uniform pore size.
- Both agarose and polyacrylamide gels can separate different biomolecules with varying size ranges. But, agarose gels are good for separating large DNA molecules. And, polyacrylamide gels are good for separating small proteins and DNA fragments.
Similarities
- Electrophoresis uses agarose and polyacrylamide-based gels to separate biomolecules (DNA, RNA, and proteins).
- Both types of gels separate biomolecules based on their size and charge.
Conclusion
Thus, agarose and polyacrylamide gels provide a support matrix to perform molecular methods. Electrophoresis uses agarose or polyacrylamide gels, as they are porous in nature. These porous gels help in separating, identifying and purifying different biomolecules.