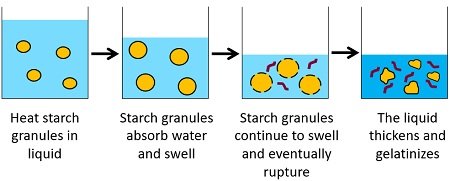
Starch gelatinization is a phenomenon in which the starch granules primarily absorb water, swell and eventually burst out to form a gel in the presence of water and heat. Gelatinization of starch is a method that requires starch (any sources like cornstarch, rice flour etc.) as a solute and water as a solvent.
Here, water serves as a plasticizer that smoothens the resulted paste. Starch is a complex polysaccharide that solubilizes or gelatinizes in warm water by forming a thick paste. Thus, it exhibits a unique property of gelatinization, and the factors like water content, temperature, pH, sugar etc., may influence the gelatinization process.
Starch gelatinization is a process of thickening used to prepare sauces, soups, puddings, custard etc. This post describes the definition, process and factors affecting starch gelatinization. You will also get to learn the meaning, structure and solubility of the starch.
Content: Starch Gelatinization
Definition of Starch Gelatinization
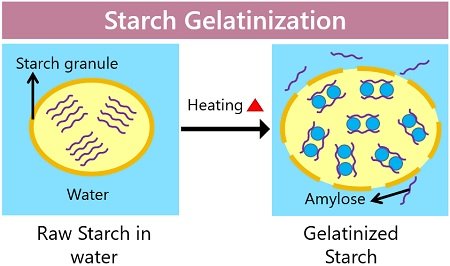
It is a process in which the solid starch granules turn into a gelated starch when dissolved in water, followed by heating the suspension. Starch granules primarily swell as the intermolecular bonding between the starch molecules breaks under high heat (50 degrees Celsius or above), resulting in more water absorption. Eventually, the starch granules burst, and they start leaching the crystalline layers as gelatinous sheets into the surrounding water.
What is Starch?
It is a polymeric carbohydrate or polysaccharide produced mostly by green plants, and it makes a significant source of diet for animals and humans. Thousands of monomeric glucose units contribute to the structure of starch. The glycosidic bond joins each glucose unit. The structure of starch includes two major components, namely amylose and amylopectin.
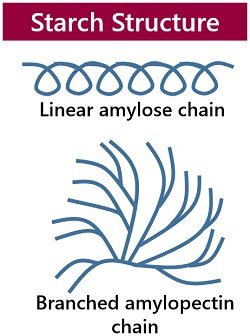
- Amylose contains linear and helical chains of alpha-D-glucose monomers. It is poorly soluble in water and slowly digestible.
- Conversely, amylopectin contains branched chains of alpha-D-glucose monomers. It is more soluble in water and more susceptible to enzyme digestion.
Unique Properties
- Starch hydrolysis yields constituent sugars.
- It remains insoluble in water.
- Starch undergoes gelatinization when heated in water.
- Gelated starch undergoes retrogradation upon cooling.
- It gives blue colour with iodine solution.
Starch Sources
Starch is produced naturally by plants, and some of the major starch sources are given hereunder.
- Cereals: Barley, corn, oats, rice, semolina, wheat, millets etc.
- Legumes: Beans, chickpeas, soybeans etc.
- Vegetables: Cassava, potatoes, taro, arrowroot, sweet potato etc.
Starch Solubility
Starch is a water-insoluble compound, thereby absorbing less water. The addition of starch into the cold or normal water form a suspension. Once the water is subjected to a temperature of 50 °C or above, the starch absorbs more water and gradually swells. By subjecting water and starch to heat, starch solubilizes and forms a thickened paste.
Process of Starch Gelatinization
It involves the following stages:
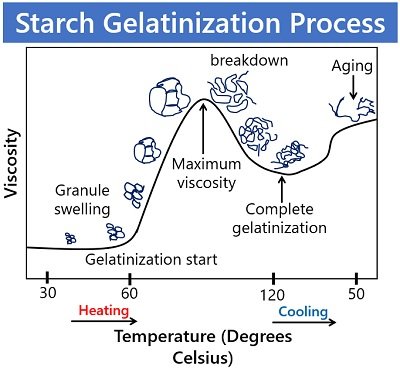
Granule Swelling
Starch granules swell when heated in water at 50 degrees Celsius or above due to the entry of water molecules into the amorphous space. On heating, the starch granules absorb water and swell irreversibly.
Double-helical Melting
Then water enters the double-helical structures of the amylopectin or the crystalline structure of starch. However, the water can not enter these crystalline regions at ambient temperature. By providing continuous heating, the temperature increases and the starch granules absorb more and more water, start bumping into each other and eventually rupture.
The water molecules break the intermolecular bonds, and the crystalline regions diffuse, resulting in the dissolution of the amylose chains. Thus, it results in the distortion of crystalline regions.
Amylose Leaching
Ultimately, the amylose chains separate into an amorphous form and leach into the surrounding water. The mixture attains the maximum viscosity as the amylose leached into the surrounding liquid causes the mixture to thicken. Complete gelatinization occurs nearly at a temperature of 96 degrees Celsius.
Ageing or Retrogradation
As the above mixture is cooled, it thickens even more and becomes a gel at about 38°C. Thus, the gelated starch gradually thickens when chilled for an extended period (hours or days). In other words, retrogradation is the recrystallization of starch in which the amylose and amylopectin chains realign again into a more crystalline structure.
In retrogradation, the water molecules trapped between the starch granules leak out, allowing the amylose/amylopectin chains to recrystallize. Amylose-amylose, amylose-amylopectin, and amylopectin-amylopectin are the three molecular associations that could form during the retrogradation.
Retrogradation is more likely to occur in a high amylose starch. The gelatinized starch may deteriorate during its storage time due to incomplete gelatinization. As a result, ungelatinized starch molecules are more susceptible to bacterial erosion than gelatinized starch granules.
Gelatinization Temperature: Here, we need to understand one important term, which is gelatinization temperature. So, it can be defined as the temperature at which the starch granules rapidly swell, lose birefringence and yield a thickened paste.
Gelatinization temperature depends upon the type of starch, the ratio of amylose and amylopectin and the water content. It varies in different starch sources, and some of the examples are mentioned hereunder.
| Sources of Starch | Gelatinization Temperature |
|---|---|
| Barley | 51-60 °C |
| Wheat | 58-64 °C |
| Potato | 60-65 °C |
| Corn | 62-72 °C |
| Rice | 68-78 °C |
Factors Affecting
- Water: Starch gelatinization requires sufficient water so that the granules can absorb water and swell. The amount of water varies depending upon the amylose and amylopectin content within the starch.
- Temperature: Generally, starch gelatinizes on heating. Large starch granules gelatinize at a relatively lower temperature like in potatoes. Oppositely, small starch granules gelatinize at a high temperature like in rice.
- pH: A pH below 4 decreases the viscosity of the gelated starch.
- Time: Heating mixture beyond the gelatinization temperature results in the loss of viscosity.
- Stirring: Initially, constant stirring is necessary to ensure uniform consistency and to prevent lump formation. But, too much agitation of the mixture may lead to premature rupturing of the starch granules.
- Sugar: It competes with starch for the availability of water, thereby decreasing the onset of gelatinization. Sometimes, it may inhibit complete gelatinization.
- Fat and Protein: They form a layer around starch granules, restricting them from absorbing water and delaying gelatinization.
Conclusion
Gelatinization is the irreversible process with respect to the shape of starch molecules. Oppositely, retrogradation is the reversible process in which the solubilized starch chains realign, forming hydrogen bonds by expelling water to form a more crystalline structure.