The qualitative analysis of lipids helps us determine the presence or absence of lipid, depending upon the colour change. Lipids are the organic biomolecules soluble in non-polar solvents like chloroform, ether, acetone etc. and insoluble in water.
In nature, different varieties of lipids show structural diversity among each other. Some common lipids are fatty acids, soaps, fats, oils, waxes, and phospholipids etc. Here, we will discuss the different qualitative methods for the analysis of lipids.
Content: Qualitative Analysis of Lipids
Definition of Qualitative Analysis of Lipids
The qualitative analysis of lipid involves some preliminary tests and specific tests to detect lipids’ presence or absence. Depending upon lipids’ reactivity with the chemical reagents, we can also classify the different groups of lipids based. Thus, the qualitative analysis of lipid is an analytical method that detects lipids by the characteristic change in the sample’s colour.
Methods for the Qualitative Analysis of Lipids
There are several methods used for the qualitative analysis of lipids and their components.
Solubility Test
It is the preliminary test that detects the presence of all lipids. Solubility test detects lipid solubility in various solvents to check whether it is miscible or immiscible in polar or non-polar solvents. Thus, it is based on the property of lipid solubility in different solvents. Lipids are readily miscible in non-polar solvents like chloroform, partially soluble in a polar solvent like ethanol and immiscible in a polar solvent like water.
Method:
- Take the lipid sample in three different test tubes by labelling it as A, B and C.
- Then, add different solvents like water, ethanol and chloroform in each test tubes A, B and C.
- Shake the tubes and allow it to stand for 1 minute.
- Check the solution for whether lipid is soluble or insoluble.
Interpretation of result:
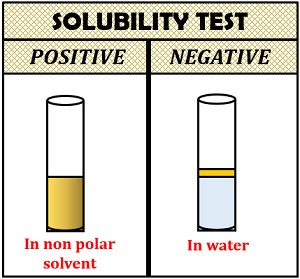
- Positive result: Lipids are soluble in a non-polar solvent, i.e. chloroform and partially soluble in ethanol which can solubilize upon heating.
- Negative result: Lipids are insoluble in a polar solvent, i.e. water.
Translucent Spot Test
A translucent spot test is also a preliminary test for the lipids, which is characterized by a translucent and greasy spot. The lipid will not wet the filter paper, unlike water. The lipids will form a greasy or translucent spot due to their greasy texture, and penetrate the filter paper. Unlike lipids, the spot of water will disappear from the paper.
Method:
- Take a filter paper.
- Add one drop of water at one end and a drop of oil or lipid at the other end.
- Observe the appearance of a translucent spot on the filter paper.
Interpretation of result:
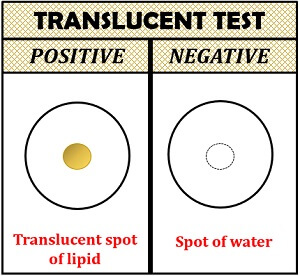
- Positive result: Translucent spot will appear on the filter paper.
- Negative result: Translucent spot will not appear on the filter paper.
Emulsification Test
Emulsification test is used to detect the presence of lipids. It is the process that stabilizes the water and oil emulsion by using the emulsifying agents. The lipid or oil in water will appear as the supernatant. The high surface tension of water develops a separate layer by adding emulsifying agents like bile salts, soap etc. Emulsifying agents emulsify the lipid, after which the lipids appear as the tiny droplets suspended in the solution.
Method:
- Take two test tubes and label it as test tube A and test tube B.
- Add oil to each of the test tubes.
- Shake the test tube and allow it to stand for about two minutes.
- Observe the test tube for the appearance of tiny droplets in the suspension of liquid.
Interpretation of result:
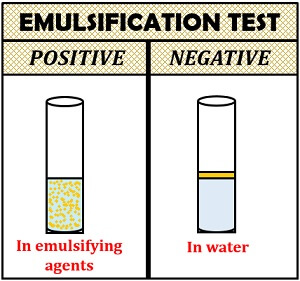
- Positive result: It gives a permanent or stable emulsion of lipid and water.
- Negative result: Oil in water emulsion will form at the top, due to the high surface tension of water.
Saponification Test
It is based on the saponification reaction, in which the triglycerides of lipid react with an alkali NaOH to produce soap and glycerol in the presence of ethanol. This reaction is also known as alkaline hydrolysis of esters.
Method:
- Take a sample of lipid in a test tube.
- Then, add strong alkali NaOH.
- Then, boil the solution in a water bath for 5 minutes.
- At last, add ethanol.
- Observe the test tube for the appearance of froth.
Interpretation of result:
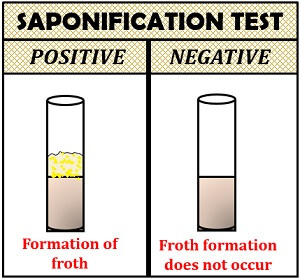
- Positive result: Froth appears in the test tube.
- Negative result: Froth does not appear in the test tube.
Sudan IV Test
Sudan IV test is used to detect the presence of lipid in a solution. This test is based upon the principle of binding and solubility of lipid in non-polar compounds. As Sudan IV is a non-polar stain, the lipid will bind with it and retain the stain’s colour by giving a red-orange colour. Sudan IV does not stain or bind to the polar compounds.
Method:
- Take 1 ml of the lipid sample in a test tube.
- Then add 1-2 drops of Sudan IV to the solution.
- Observe the tubes for the appearance of red-orange colour in the solution.
Interpretation of result:
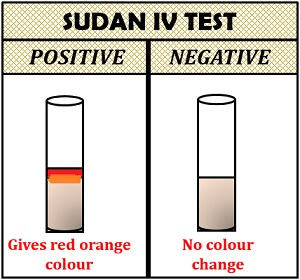
- Positive result: Gives red-orange colour to the solution.
- Negative result: The solution of the colour will remain unchanged.
Acrolein Test
Acrolein test is used to detect the presence of glycerol and fat. This test is based on the dehydration reaction, in which the water molecules are removed from the glycerol by adding reagent potassium hydrogen sulphate. The reaction between glycerol and potassium hydrogen sulphate results in acrolein formation, which is characterized physically by the release of the pungent smell.
Method:
- Take 1 ml of the lipid sample in a test tube.
- Add crystals of potassium hydrogen sulphate.
- Heat the solution for a few minutes.
- Smell the test tube for the pungent smell.
Interpretation of result:
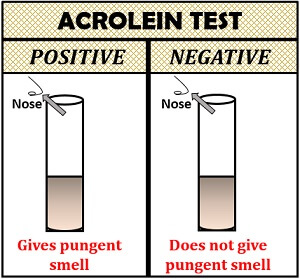
- Positive result: If glycerol present in the sample, it will give a pungent smell.
- Negative result: If glycerol is absent in a sample, it will not produce a pungent smell.
Dichromate Test
Dichromate test is also used to detect the presence of glycerol. It is based on the principle of an oxidation reaction. In this, glycerol and dichromate ions react to give a brown colour to the solution. Then, the chromic ions oxidize the glycerol and reduce into chromous ions by giving a blue colour to the solution in the presence of nitric acid.
Method:
- Take 2-3 ml of a sample in a test tube.
- Then, add a few drops of 5% potassium dichromate solution.
- After that, add 5 ml of concentrated nitric acid.
- Observe the test tube for the appearance of a blue colour.
Interpretation of result:
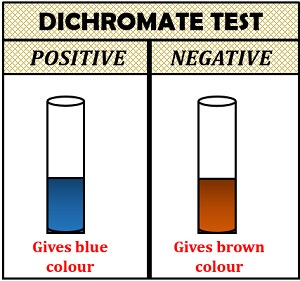
- Positive result: If the colour of the solution changes from brown to blue, it indicates glycerol.
- Negative result: In this, the brown colour will not change into blue.
Tests for the Free Fatty Acids
This is based on the neutralization reaction where the alkali neutralizes by adding free fatty acids into the lipids.
Method:
- Add phenolphthalein solution in a test tube.
- Then, add dilute alkali to the above solution (gives a pink colour).
- At last, add a lipid sample.
- Observe the tube for the disappearance of the pink colour after the addition of lipid.
Interpretation of result:
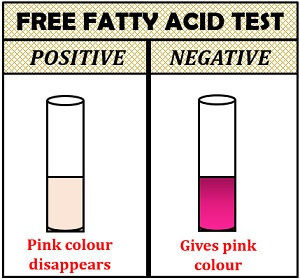
- Positive result: If the pink colour disappears by adding the lipid sample, it indicates free fatty acids in the sample.
- Negative test: The pink colour will not disappear.
Unsaturation Test
Unsaturation test is used to detect the unsaturated fatty acids or double bond in a lipid sample. All the neutral fat contains glycerides of fatty acids. Double bonds are found in the structure of unsaturated fatty acids, which becomes saturated by taking up either bromine or iodine. If the lipid contains more unsaturated fatty acids or more double bonds, it will take more iodine.
Method:
- Take 5 ml of chloroform and 5 ml of Huble’s iodine reagent in a beaker, giving pink colour to the solution.
- Add lipid sample drop by drop and shake vigorously, until pink colour disappears.
- Count the number of drops added to chloroform and Huble’s iodine solution until pink colour disappears. The number of drops determines the taking up of iodine by the unsaturated fatty acid of lipids.
Interpretation of result:
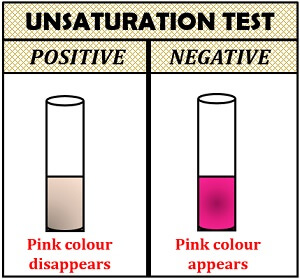
- Positive result: Pink colour will disappear by the addition of unsaturated fatty acids.
- Negative result: Pink colour will not disappear.
Burchard Test
Burchard test was first given by the scientist Liebmann to detect the presence of cholesterol. Cholesterol reacts with the strong concentrated acid, i.e. sulphuric acid and acetic anhydride. Sulphuric acid and acetic anhydride act as a dehydrating and oxidizing agent.
Method:
- Take crystals of cholesterol in a test tube.
- Then, add 2 ml of chloroform to dissolve the cholesterol.
- Add 10 drops of acetic anhydride in a solution and 2-3 drops of concentrated sulphuric acid.
- Observe the test tube for the appearance of a bluish-green colour.
Interpretation of result:
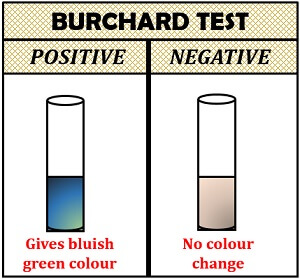
- Positive result: It indicates cholesterol in a sample by giving bluish-green colour to the solution.
- Negative result: The colour of the solution will not change.
Conclusion
We can conclude that lipids’ qualitative study is useful in classifying heterogenous lipids (like wax, steroids, triglycerides, fats, etc.) based on the properties like solubility, emulsification, oxidation etc. The presence of lipids is qualitatively characterized by the characteristic change in colour, smell and froth formation.
Really amazing article. Tomorrow is my paper…
This article is very interesting.
Thank you so much. It was really useful for my biochemistry project.
Very accurately and well-explained notes. Special Thanks from Fateh Singh!