The separation of plant pigments by paper chromatography is an analysis of pigment molecules of the given plant. Chromatography refers to colour writing. This method separates molecules based on size, density and absorption capacity.
Chromatography depends upon absorption and capillarity. The absorbent paper holds the substance by absorption. Capillarity pulls the substance up the absorbent medium at different rates.
Separated pigments show up as coloured streaks. In paper chromatography, the coloured bands separate on the absorbent paper. Chlorophylls, anthocyanins, carotenoids, and betalains are the four plant pigments.
This post discusses the steps of separating plant pigments through paper chromatography. Also, you will get to know the observation table and the calculation of the Rf value.
Content: Separation of Plant Pigments by Paper Chromatography
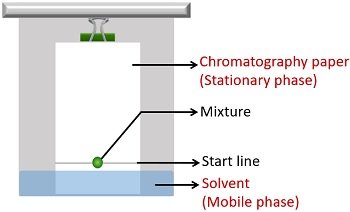
Paper Chromatography
It is the simplest chromatography method given by Christian Friedrich Schonbein in 1865. Paper chromatography uses filter paper with uniform porosity and high resolution.
The mixtures in compounds have different solubilities. For this reason, they get separated distinctly between the stationary and running phase.
- The mobile phase is a combination of non-polar organic solvents. The solvent runs up the stationary phase via capillary movement.
- The stationary phase is polar inorganic solvent, i.e. water. Here, the absorbent paper supports the stationary phase, i.e. water.
Plant Pigments
Plant pigments are coloured organic substances derived from plants. Pigments absorb visible radiation between 380 nm (violet) and 760 nm (red).
They give colour to stems, leaves, flowers, and fruits. Also, they regulate processes like photosynthesis, growth, and development.
Plants produce various forms of pigments. Based on origin, function and water solubility, plant pigments are grouped into:
- Chlorophylls (green)
- Carotenoids (yellow, orange-red)
- Anthocyanins (red to blue, depending on pH)
- Betalains (red or yellow)
Chlorophyll: It is a green photosynthetic pigment. Chlorophyll a and b are present within the chloroplasts of plants. Because of the phytol side chain, they are water-repelling. Their structure resembles haemoglobin. But, they contain magnesium as a central metal instead of iron.
Carotenoids: These are yellow to yellow-orange coloured pigments. Also, they are very long water-repelling pigments. Carotenoids are present within the plastids or chromoplasts of plants.
Anthocyanins: These appear as red coloured pigments in vacuoles of plant cells. Anthocyanins are water-soluble pigments. They give pink-red colour to the petals, fruits and leaves.
Betalains: These are tyrosine derived water-soluble pigments in plants. Betacyanins (red-violet) and betaxanthins (yellow-orange) are the two pigments coming in this category. They are present in vacuoles of plant cells.
You can separate all the above pigments using paper chromatography.
Video: Separation of Plant Pigments
Steps of Plant Pigment Separation
Let us discuss the procedure of separating plant pigments by paper chromatography. The diagram below indicates the major steps of pigment separation.
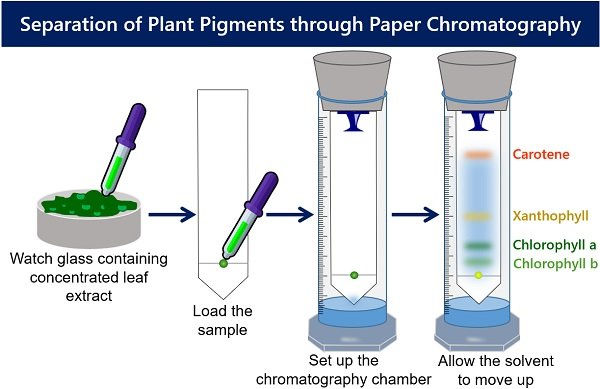 First, the concentrated leaf extract is prepared. After that, load the sample on the chromatography paper. Then, keep a paper containing sample spot inside the chromatography chamber containing solvent. At last, allow the solvent to move up to visualize the coloured spots.
First, the concentrated leaf extract is prepared. After that, load the sample on the chromatography paper. Then, keep a paper containing sample spot inside the chromatography chamber containing solvent. At last, allow the solvent to move up to visualize the coloured spots.
Preparation of Concentrated Leaf Extract
This stage requires the following goods as illustrated in the picture below:
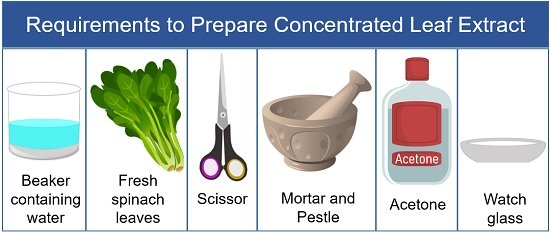
- Wash spinach leaves in distilled water.
- Then take out the spinach leaves and allow the moisture to dry out.
- After that, take a scissor and cut the leaves into the mortar.
- Take a little volume of acetone into the mortar.
Note: Acetone is used instead of water to mash the leaves because it is less polar than the water. This allows a high resolution of the molecules in the sample between the absorbent paper. - Then, grind spinach leaves using a pestle until liquid paste forms.
Note: The liquid in the crushed leaf paste is the pigment extract. - After that, take out the mixture into the watch glass or Petri dish.
Load the Leaf Extract onto Absorbent Paper
To load the sample, you require the materials illustrated in the picture below:

- Take Whatman filter paper and draw a line above 2 cm from the bottom margin. You can use a pencil and scale to draw a fainted line.
Note: A pencil is used because pencil marks are insoluble in the solvent. - Then, cut the filter paper to make a conical edge from the line drawn towards the margin end. You can use a scissor to cut the Whatman filter paper.
Note: The conical end at the bottom of the filter paper results in better separation. - Put a drop of leaf extract on the centre of a line drawn on the absorbent paper.
- Then, at the same time dry the absorbent paper.
- Repeat the above two steps many times so that the spot becomes concentrated enough.
Setup the Chromatography Chamber
This stage requires the materials displayed in the picture below:
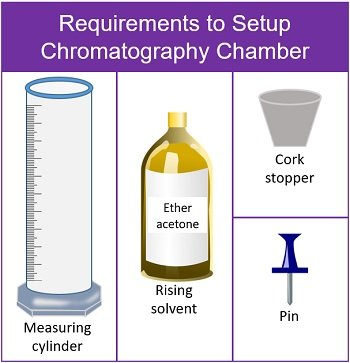
- Take a clean measuring cylinder and add rising solvent (ether acetone) up to 4 ml.
- Bend the strip of paper from the top. Then, using a pushpin attach the paper to the bottom of the cork.
- Adjust the length of the paper. The absorbent paper should not touch the surface of the measuring cylinder.
- After that, allow the solvent to move up the absorbent paper.
- When the solvent front has stopped moving, remove the paper.
- Allow it to dry for a while until the colours completely elute from the paper.
- At last, mark the front edge travelled by each pigment.
Observation
Over the dried paper strip, you will see four different bands. Different colour streaks form because of different affinities with the mobile phase (solvent).
- The carotene pigment appears at the top as a yellow-orange band.
- A yellowish band appears below the carotene, which indicates xanthophyll pigment.
- Then a dark green band represents the chlorophyll-a pigment.
- The chlorophyll-b pigment appears at the bottom as a light green band.
Observation Table
Measure the distance from the starting point to the centre of each coloured band using a scale. Then measure the entire distance travelled by the solvent.
| Band Colour | Plant Pigment | Distance from sample spot (cm) | Solvent front (cm) | Rf Value |
|---|---|---|---|---|
| Light green | Chlorophyll-b | 2 cm | 10 cm | 0.2 |
| Dark green | Chlorophyll-a | 3.7 cm | 10 cm | 0.37 |
| Yellow | Xanthophyll | 5.6 cm | 10 cm | 0.56 |
| Yellow-orange | Carotene | 9 cm | 10 cm | 0.9 |
Calculation
Calculate Rf values for each pigment. Rf stands for retardation or retention factor. You can record the Rf value by knowing the distance analyte travelled by the distance solvent travelled.
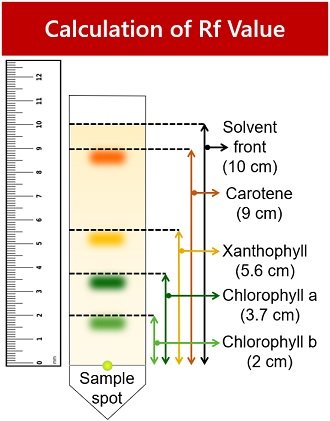
1. Light green spot indicates chlorophyll-b pigment.
- Rf value= Distance chlorophyll-b travelled / Distance solvent travelled = 2/10 = 0.2
2. Dark green spot represents chlorophyll-a pigment.
- Rf value= Distance chlorophyll-a travelled / Distance solvent travelled = 3.7/10 = 0.37
3. The yellow band represents xanthophyll pigment.
- Rf value= Distance xanthophyll travelled / Distance solvent travelled = 5.6/10 = 0.56
4. The yellow-orange band indicates carotene pigment.
- Rf value= Distance carotene travelled / Distance solvent travelled = 9/10 = 0.9
Factors affecting the Rf values of a particular analyte are:
- Stationary phase
- The concentration of the stationary phase
- Mobile phase
- The concentration of the mobile phase
- Temperature
The Rf value of compounds in the mixture differs by any changes in the concentration of stationary and mobile phases.
Temperature affects the solvent capillary movement and the analyte’s solubility in the solvent. Rf value is independent of the sample concentration. Its value is always positive.
Nice experiment and understanding.