General properties of enzyme include all the characteristics (physical and chemical), through which we could define the physical and chemical nature of an enzyme. Enzymes act like a functional biocatalyst that only cause catalysis of distinct substrates into particular products and allow the reaction to occur at an increased rate without being consumed.
Thus, the enzyme’s primary function is to accelerate the reaction rate. Enzymes neither affect the nature of products formed nor undergo any changes by the reaction catalyzed. Only the active site of an enzyme goes through certain conformational changes during the substrate binding.
Upon reaction catalysis, an enzyme turns back to its original form and becomes available to participate in the new biochemical reactions. In this post, we will discuss the reasons why enzymes are considered biocatalysts along with the enzyme’s general properties. You could also learn the physical and chemical nature of an enzyme in this context.
Content: General Properties of Enzyme
- Introduction to Enzyme
- Enzymes as Biocatalysts
- General Properties
- Physical Properties
- Chemical Properties
Introduction to Enzyme
Wilhelm Kuhne introduced the term enzyme in 1878. Enzymes are biomolecules that are highly specific. All metabolic processes in our body need enzymes to catalyze reaction at a faster rate. Enzymes are naturally produced by living cells, unlike catalysts.
The enzyme’s activity decreases remarkably under high heat. The majority of enzymes are globular proteins, while some RNA molecules. Enzymes are larger than substrates. Enzymes reside within the protoplasm as hydrophilic colloids.
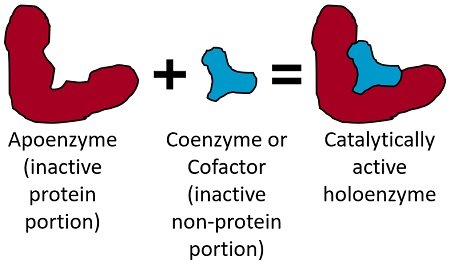
Apoenzyme constitutes the enzyme’s inactive protein portion. Conversely, coenzymes (organic molecules) and cofactors (inorganic ions) make up the inactive, non-protein component of an enzyme. Thus, the main difference between cofactor and coenzyme is the chemical nature. When united, both form the catalytically active holoenzyme (complete enzyme).
Enzyme tends to attain a shape complementary to the transition state structure. Therefore, specificity is due to the structural relativeness between enzyme and substrate.
Enzymes as Biocatalysts
Reasons that why enzymes are regarded as “Biocatalysts” are as follows:
- Like catalysts, enzymes speed up the reaction rate by reducing the transition time between the substrate and product.
- As catalysts, enzymes also regulate reaction-specificity, in which only specific substrate adhere to an enzyme’s active site to bring product formation.
- Similar to the function of catalysts, enzymes only take part in the biochemical reaction without being consumed or altering the equilibrium state.
- Like catalysts, enzymes influence or initiate the biochemical reaction by lowering the activation energy and increase the transition energy of substrate into product.
General Properties of Enzyme
The general properties of enzyme include the following characteristics:
- Enzymes initiate and accelerate the reaction.
- The activity of an enzyme is pH-specific.
- Enzymes can catalyze reactions in a forward and reverse manner, but do not decide the direction of the biochemical pathway.
- An enzyme possesses a specialized region (active site), to which substrate specifically interacts to form desired products.
- Under high heat, temperature and varying pH, an enzyme becomes unstable.
- Enzymes are proteinaceous possessing properties characteristic to proteins.
- A small amount of enzyme is required to bring catalysis of substrates.
- Enzyme’s activity typically shows absolute, relative, group and stereo-specificities.
- Some enzymes are regulatory in function.
- Its primary function is to minimize the activation energy.
- The enzymes remain unaltered during and after the product formation, or they can be reconsumed.
- An enzyme may possess an allosteric site besides an active site, to which cofactors or regulators interact.
- Enzymes are soluble in water and NaCl.
Physical Properties of Enzyme
We will study the following physical characteristics of enzyme, mentioned in a figure below.
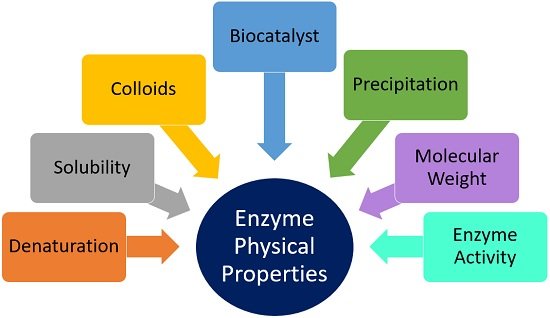
Biocatalyst Nature
Enzymes serve as a biocatalyst, and the reasons we have discussed earlier in this post.
Enzyme Activity
The mechanism of enzyme action strongly depends upon the factors like temperature, pH, and concentration of enzyme and substrate. Enzymes show the highest activity at optimum temperature (37 degrees Celsius) and pH (7.2). A low concentration of enzyme and substrate will slow down the enzymatic reaction.
On the other hand, a higher concentration of enzyme will result in faster enzymatic activity, as more substrates will interact with the enzyme’s active site to bring product formation.
Once the reaction velocity reaches a maximum value, there would not be any changes in the enzymatic reaction even after the addition of enzyme and substrate.
Colloidal Nature
Enzymes behave as colloids due to their large size or high molecular weight. As a result, the enzymes have a little or no tendency to dialyze or cross the semi-permeable membrane.
Coenzymes are the inactive non-protein component that has a low molecular weight and highly dialyzable.
Enzyme Precipitation
Acidic and alkaline solution can cause enzyme precipitation, as the enzymes are amphoteric (possess amino and carboxylic acid group towards the end of the chain). Ethanol and a high concentration of inorganic salts like ammonium sulphate facilitate enzyme precipitation.
Molecular Weight
Enzymes are large protein biomolecules possessing a polypeptide chain of various amino acid sequence. Nearly 200 to 300 peptide bonds hold the amino acids together. Therefore, enzymes have a high molecular weight.
Enzyme Solubility
Enzymes are soluble in water, NaCl, diluted glycerol and alcohol.
Enzyme Denaturation
High heat (above 40 degrees Celsius) and alternations in the pH (too low and too high), heavy metals, and high salt concentration etc., denatures the enzyme by breaking the intra and inter-molecular noncovalent bonds. It distorts the enzyme’s shape and active site and finally results in the loss of enzyme activity.
Chemical Properties of Enzyme
We will study the following chemical features of an enzyme, mentioned in a figure below.
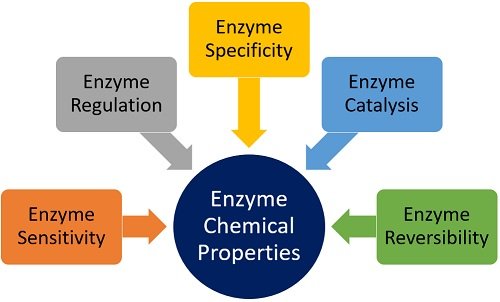
Enzyme Catalysis
Enzymes are biological catalysts, which possess high catalytic efficiency. They can transform about 100-10,000 substrates/second. The reactions catalyzed by the enzymes show 103-108 times faster reaction rate in comparison to the uncatalyzed reaction.
Enzymes do not cause alternations in the equilibrium constant and increase the conversion rate. The number of substrate molecule gives the product is called the turn over number.
Enzyme Regulation
The enzyme function can be regulated allosterically. Other than the enzyme’s active site, there is another site, to which a regulatory molecule binds.
A regulatory molecule may function as an activator or inhibitor. It binds to the region called the allosteric site. Enzymes that need metabolic regulators are called allosteric enzymes.
The allosteric inhibitor binds to an enzyme’s active site can cause slight alternations in its shape that restrict substrate-binding. On the other hand, allosteric activators increase the function or efficiency of an enzyme.
Enzyme Specificity
Enzymes are reaction-specific, possessing four distinct types of specificity.
- Absolute specificity: An enzyme catalyzes only one reaction, or it binds to a single substrate. For instance, maltase acts on maltose.
- Group specificity: The enzymes act upon the substrates that have specific functional groups. For example, Pepsin hydrolyzes the peptide bond of phenylalanine.
- Relative or bond specificity: Enzymes act on the reactant molecules having a similar bond and structural conformational. For example, amylase hydrolyzes alpha-1,4 glycosidic linkage in starch and glycogen. It has a low specificity.
- Stereochemical specificity: Enzymes form a complex with the particular steric or optical isomers. L-amino acid oxidase acts specifically on L-amino acid. It has high specificity.
Enzyme Sensitivity
Enzymes work best at optimum temperature and pH. Enzyme’s activity is most significant at an optimum pH of 7.2 but generally ranges between pH 6 to 9. 37 degrees Celsius is an optimum temperature required for the enzyme to function.
Temperature above 37.5 degrees Celsius and any alternations in the pH may not only reduce the effectiveness of the enzyme but also results in enzyme denaturation. As a result of denaturation, the intra and intermolecular bonds between the enzyme molecules break.
Enzyme Reversibility
Enzymes are the biomolecules that catalyze various metabolic reactions, including catabolic and anabolic reactions. In simple words, an enzyme can synthesize (build up new molecules or products) and decompose or break down the different products.
The majority of the enzymatic reactions are reversible, which means an enzyme can catalyze biochemical reactions in a forward and reverse direction. Digestive enzymes catalyze reactions in one way.