The difference between cofactor and coenzyme is mainly characterized based on chemical nature and function. Cofactors constitute a large group of helper molecules (inorganic or organic). Conversely, cofactors are small organic molecules.
Coenzymes significantly act as carrier materials to convert the inactive protein (the apoenzyme) into the active form (holoenzyme). In contrast, cofactors serve as biocatalysts that only fasten the enzymatic reaction inside a cell.
Both cofactor and coenzyme are important terms to study the general properties of an enzyme. It is important to note that cofactors or coenzymes only attach to the types of conjugated enzymes containing a non-protein region.
Hence, simple enzymes containing amino acids do not require any additional carriers to show their catalytic activity. This post discusses the key differences between the cofactor and coenzyme, along with the comparison chart, definitions and examples of the two.
Content: Cofactor Vs Coenzyme
Comparison Chart
| Properties | Cofactor | Coenzyme |
|---|---|---|
| Alternative names | Helper molecules or accessory molecules | Co-substrate or secondary substrate |
| Definition | Cofactors refer to the non-protein helper molecules required for the activity of apoenzymes or enzymes made of conjugated proteins, which may include simple metal ions or complex organic groups | Coenzymes refer to the inactive non-protein organic co-substrates (without a protein part or apoenzyme) that directly participate in the enzyme catalysis |
| Chemical nature | Cofactors can be organic and inorganic molecules | Cofactors are organic molecules |
| Association with an enzyme | It can covalently or non-covalently associate with an apoenzyme | It binds loosely or non-covalently with an apoenzyme |
| Separation | Separation of cofactors can be easy or difficult (separates only after enzyme denaturation) | Coenzymes are attached transiently to an apoenzyme and can be easily detachable |
| Dialysability | Few cofactors are dialysable, while others are non-dialysable | It is dialysable |
| Classification | It is classified into two types based on the enzymatic activity, namely inorganic and organic cofactors | It is a subtype of cofactor that comes under the category of organic cofactors |
| Function | It functions as the helper molecule that fastens the enzymatic reaction | It functions as the substrate shuttle that helps in translocating atoms or groups |
| Integral part | A cofactor is a collective term that represents activator metal ions, coenzymes, prosthetic groups necessary for an inactive enzyme to function | The integral part of the coenzymes are vitamins |
Definition of Cofactor
It refers to the small, non-protein, helper or accessory molecule necessary to bring an inactive apoenzyme to an active state termed holoenzyme or complete enzyme. Apoenzymes are conjugated proteins, which require an additional factor to act like a functional or catalytically active enzyme.
An enzyme contains an active site where the specific substrate binds firmly. Oppositely, the allosteric site is the region where the allosteric activators and inhibitors can bind particularly to accelerate or inhibit the enzymatic activity. Cofactors can be inorganic (includes metals ions) and organic (includes coenzymes and prosthetic groups).
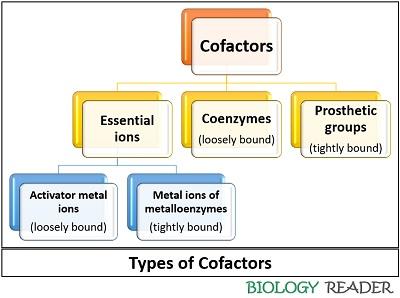
According to the chemical nature and association with an enzyme, the cofactors are classified into two types.
- A large group of metal ions like (Mg 2+, Cu +, Mn 2+) are inorganic cofactors that serve as essential trace elements in our diet. Enzymes that activates in association with metal ions are called metal activated enzymes or metalloenzymes.
- In some cases, an enzyme needs to be allosterically regulated by the binding of certain inorganic elements. Here, the inorganic elements are generally effector molecules but not regarded as cofactors. For instance, calcium participates in the allosteric regulation of nitric oxide synthase, adenylate kinase etc.
- Organic cofactors include coenzymes and prosthetic groups, which we will discuss below in the definition of coenzyme.
Definition of Coenzyme
It refers to the co-substrate or secondary substrate that constitutes a group of inactive, non-protein and small organic molecules of low molecular weight (< 1000Da). They directly participate in the enzyme catalytic reactions.
The coenzyme is a subtype of cofactor molecules, which are organic in nature and assists in binding a substrate molecule to an enzyme’s active site. Coenzymes bind weakly to the inactive protein or apoenzyme, which can be easily separated by dialysis. It plays a conclusive role in an inactive enzyme to function.
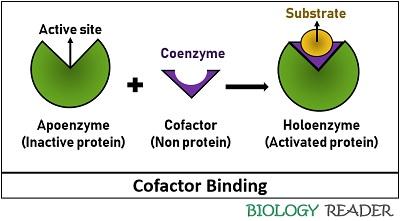
As we have discussed, some enzymes need a specific carrier or molecules to catalyse a reaction. Thus, the coenzyme works as a co-substrate that primarily binds with the substrate molecules, undergoes some alternation during enzyme activity, and later regenerates or functions as a recyclable shuttle.
Prosthetic groups are complex organic groups that bind covalently with the protein. Their separation from an enzyme is difficult, or they only separate by the enzyme denaturation. For instance, heme is a prosthetic group containing an iron atom in the haemoglobin molecule.
Cofactor Examples
There are few examples of the enzymes and their cofactors:
- Fe2+ or Fe3+: Catalase, Peroxidase etc.
- Cu2+: Cytochrome oxidase, superoxide dismutase etc.
- Zn2+: Alcohol dehydrogenase, Carboxypeptidase etc.
- Mg2+: Hexokinase, Enolase, Glucose 6-P
- Mn2+: Arginase, Enolase, Pyruvate carboxylase
- K+: Pyruvate kinase
- Ni2+: Urease
- Mo: Dinitrogenase, Xanthine oxidase
- Se: Glutathione peroxidase
Coenzyme Examples
There are few examples of coenzymes of vitamin B-complex:
- Thiamine (B1 vitamin): Thiamine pyrophosphate is the coenzyme of thiamine precursor of vitamin B1 that participates in the decarboxylation, aldehyde group transfer etc.
- Riboflavin (B2 vitamin): FAD and FMN are the flavin molecules that acts as a coenzyme of vitamin B2, which actively participate in the redox reactions.
- Pyridoxine (B6): Pyridoxal phosphate is a coenzyme of pyridoxine precursor of vitamin B6 that carries out amino group transfer.
Key Differences Between Cofactor and Coenzyme
- Helper or accessory molecules are the alternative names for the term cofactor, and co-substrate or secondary substrate are the alternative names for the term coenzyme.
- Cofactors refer to the non-protein helper molecules required for the activity of apoenzymes or enzymes made of conjugated proteins, which may include simple metal ions or complex organic groups. Coenzymes refer to the inactive non-protein organic co-substrates (without a protein part or apoenzyme) that directly participate in the enzyme catalysis.
- Cofactors are classified into two types based on the enzymatic activity, namely inorganic and organic cofactors. The inorganic cofactors include metal ions, while organic cofactors include coenzymes and prosthetic groups. Thus, the coenzyme is a subtype of cofactors.
- Cofactors constitute a broad group of accessory elements, out of which some may associate covalently or non-covalently with an apoenzyme. Coenzymes are carrier molecules that join transiently or non-covalently with an apoenzyme.
- The separation of cofactors from an enzyme is sometimes easy or difficult (only separates after enzyme denaturation). As the coenzymes are transiently attached to the enzyme complex, they are easily separable.
- Dialysability is a property of the ions or molecules through which they can diffuse down a semipermeable membrane or separated by dialysis. Few cofactors are dialysable (like coenzymes and metal ions), whereas others are non-dialysable.
- The function of the cofactor is to fasten the enzymatic reaction. Coenzymes work as substrate shuttles that help in translocating atoms or groups from one place to the other inside a cell.
Conclusion
Therefore, we can conclude that the cofactor is a collective term that includes inorganic metal ions, organic compounds (coenzymes) and organic prosthetic groups. Coenzymes and cofactors are the additional factors that facilitate enzyme catalysis by binding with the inactive protein that alone can not convert substrate into product.