Retrogradation of starch is just opposite of the starch gelatinization. Here, the term retrogradation denotes the reversal mechanism. The gelation of starch contains disaggregated starch molecules.
But, the disaggregated amylose and amylopectin chains realign during retrogradation of a starch gel. We could see the retrograde starch after a long-term cooling or storage of the starch gel.
Boussingault introduced this process in 1852. Freezing is a factor aggravating the retrogradation process. Upon cooling, the amylose and amylopectin chains retrograde in a parallel fashion. Later on, the hydrogen bonds reform between the chains.
Then, it leads to the formation of a compact or crystalline structure of starch. But, the crystallinity of retrograded starch is not the same as the native starch granule.
This post describes the meaning, key points, examples and factors affecting starch retrogradation. We will also discuss how does starch retrogradation occur?
Content: Retrogradation of Starch
- Meaning
- How Does Retrogradation Occurs?
- Key Points
- Examples
- Factors Affecting
- How to Prevent Retrogradation?
Meaning of Starch Retrogradation
Starch retrogradation refers to the recrystallization of starch upon cooling the starch gel. The process is thermoreversible. It involves the realignment of amylose chains. Also, it involves the aggregation of amylopectin chains into a more crystalline form.
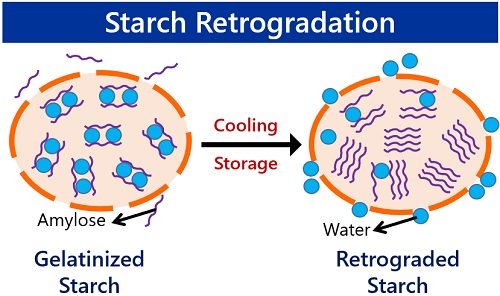
Or, we can say it is like a syneresis of starch gel. Syneresis is the discharge of a liquid or water from a gel. Likewise, water expels out of the starch granules during starch retrogradation.
How Does Retrogradation Occurs?
Retrogradation is an ageing process of viscous or gelatinized starch. Upon ageing of the gelatinized substance, amylose and amylopectin reassociate or recrystallize. Starch retrogradation has the following two stages:
1. Short-term retrogradation
It includes linear amylose chains. Amylose recombines in an irreversible manner to produce a crystal nucleus. It is a rapid process, as the reassociation becomes faster due to the linear structure of amylose. Emulsifiers may reduce the initial firmness or amylose retrogradation.
2. Long-term retrogradation
It includes branched and more complex amylopectin chains. Unlike amylose, amylopectin retrogradation takes a long time to crystallize. It takes several days or weeks. This is because of the branched structure of the amylopectin. The introduction of enzymes reduces the long-term firmness or amylopectin retrogradation.
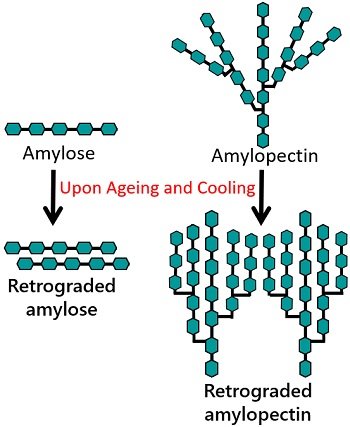
Thus, the retrogradation rate differs in amylose and amylopectin polysaccharides. Amylose retrogradation is a faster event than amylopectin retrogradation.
Note: Amylose has high crystallinity and is susceptible to retrogradation. In contrast, amylopectin crystallizes only outside the globule and shows slow retrogradation.
Key Points
- Starch is a homopolysaccharide. It comprises two α-d-glucan chains, amylose and amylopectin. The starch structure and composition varies, depending on the type of plant species.
- Amylose exists as a linear chain. It contains about 500-2000 glucose units.
- In contrast, amylopectin has a branched structure. It contains about 1,000,000 glucose units.
- Starch retrogradation is the starch’s physicochemical property. It is the stage after starch gelatinization. By cooling or ageing the starch gel for a longer period, retrogradation of starch units occur. During retrogradation:
- The glucose units of amylose combine to produce a double-helix.
- The glucose units of amylopectin recrystallize through a combination of its small chains.
- The above interaction results in a more ordered or crystalline form. Hydrogen-bond reforms within such units and holds up these units together. Also, it accounts for the crystalline structure of the retrograded starch.
- Starch retrogradation involves a series of physical changes like:
- Increased viscosity
- Turbidity of pastes
- Gel formation
- Exudation of water (syneresis)
- The free starch molecules form a network by forming ordered structures. These reorganized structures differ from the crystalline structure of the native starch granules.
- Retrograded starch is less digestible. This is because digestive enzymes have difficulty in breaking the starch crystals.
Retrogradation of Starch Examples
Here, we will take the example of starch retrogradation in bread and rice. Also, we will study the changes in starch granules till the retrogradation process:
Bread Retrogradation
In the dough stage, starch molecules are in crystalline form. Here, the linear and branched chains of amylose and amylopectin appears
Then, the raw bread dough gelatinizes at about 150° C. The starch absorbs water, swells, and becomes semi-firm during this stage. After baking, the loaf is taken out of the oven.
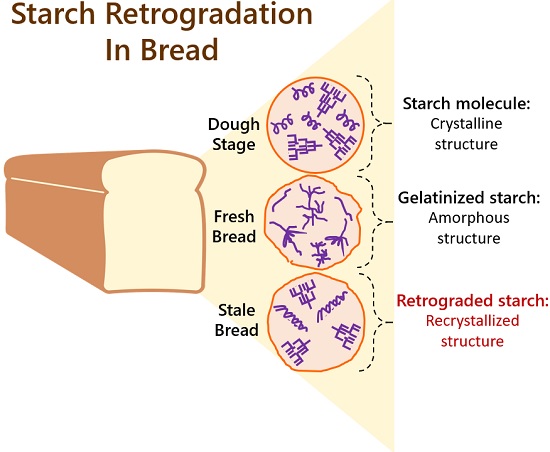
Upon ageing and cooling below the gelatinization temperature, the starch molecules recrystallize. The starch expels water, contract, and becomes harder during this stage.
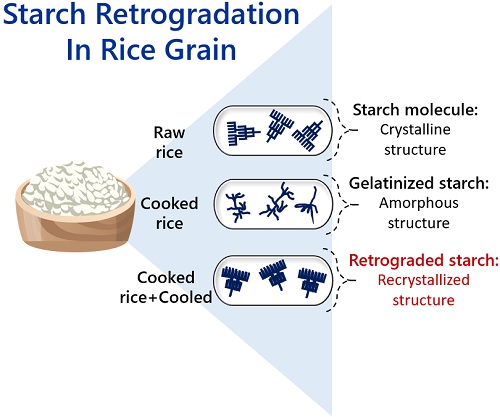
Rice retrogradation
Starch molecules in raw rice have a crystalline structure. By cooking rice in water, the starch molecules gelatinize. The rice grain swells by absorbing water. Starch molecules rearrange into a crystalline structure once you refrigerate or age the cooked rice.
Factors Affecting
Factors affecting retrogradation involve:
1. Type of starch: The retrogradation rate differs depending upon the starch type and composition. Starch with high amylose concentration retrogrades faster due to linear chain structure.
2. Length of the amylopectin chains: Amylopectin with short branches constitute the crystalline region. More amylopectin concentration in starch granules results in greater crystallinity.
3. Proteins: These serve as emulsifiers. They have a negative effect on the retrogradation properties of the paste. Protein-starch complexes retard retrogradation during storage.
4. Lipids: Monoglycerides are lipids that interact with free amylose. Free amylose forms a complex at a concentration of monoglycerides >1%. Also, amylopectin-lipid complex forms. This interaction obstructs the amylopectin crystal formation. And, resulting in a decrease in retrogradation.
5. Physical modification: Repeated freezing of the starch gel aggravates retrogradation. Thus, the resulting starch formed is RS-III (resistant starch). RS-III is resistant to amylase digestion.
6. Chemical modification: A chemical process like acetylation reduces the retrogradation tendency of starch. Reagents like anhydrous acetic acid or vinyl acetate cause esterification of native starch. The process requires an alkaline catalyst (NaOH, KOH, Ca (OH)2, or Na2CO3).
How to Prevent Retrogradation?
Some measures can prevent retrogradation.
- Chemical starch modifications like hydrolysis and esterification produce starches resistant to retrogradation.
- Adding fat and protein as an emulsifier can also reduce retrogradation.
- Sodium chloride and sodium nitrate are also some reagents preventing starch retrogradation.
- Sugar molecules also delay retrogradation. They interact with starch chains to stabilize the amorphous regions of the granule.
By preventing retrogradation, we can increase the longevity of food items.