Membrane proteins are the binding proteins that mediate the conduction of ions or molecules into and out of the cell membrane. Integral, peripheral and lipid-anchored are the three typical membrane proteins.
The membrane protein is the principal constituent of the cell membrane that contributes to the plasma membrane structure. The union of membrane proteins and the phospholipid bilayer cell membrane could be temporary or permanent.
A biological layer has more than hundreds of protein at defined orientation. In this post, we will study the definition, assembling, types and functions of membrane proteins.
Content: Membrane Proteins
Definition of Membrane Proteins
Membrane protein refers to the constitutive or non-constitutive protein, which unite with the phospholipid bilayer membrane at different locations by entirely or partially spanning the plasmolemma.
“Membrane sidedness” is due to the different location of various membrane proteins in the biological cell membrane, which causes asymmetry of the cell membrane. Some membrane proteins communicate with the extracellular substances, while some interact with the inner protoplasmic material.
Assembling of Membrane Proteins
We all know the proteins form through mRNA translation by the association of ribosome and transfer RNA. The ribosomes synthesize either a sequence of true signal or non-cleavable TM segment from their N-terminal.
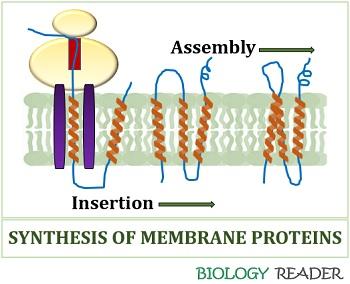
Then, the hydrophobic segment of amino acid residues travels through the ribosomal tunnel towards the exit site. Translocon refers to the protein complex within the cell membrane that provides a ribosomal tunnel to release and insert newly synthesized proteins.
The ribosomes process the release of Tm segments relative to the encoded mRNA sequence. Signal receptor particles (SRPs) bind with the incoming polypeptide chain complex, accommodating the signal sequence in the alpha-helical conformation and halts translation.
The SR receptors interact with the ribosomes and SRPs and cause a conformational change in SRPs, allowing the transfer of the nascent ribosome chain. After the protein synthesis, the ribosome detaches from the translocon, and the released membrane proteins assume their final 3D conformation.
Types of Membrane Protein
Based on location and structure, the membrane proteins are typically classified into the following groups:
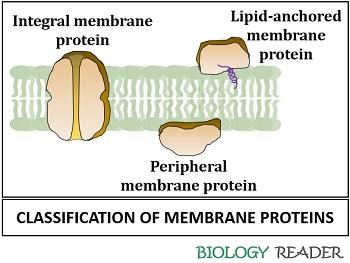
Integral Proteins
They refer to the intrinsic proteins, which completely spans the phospholipid bilayer membrane. Transmembrane or integral proteins have domains within the cytoplasm and towards the extracellular ends of the lipid bilayer.
They either have multiple spanning segments and contribute about 25-30% of all the encoded proteins. Integral proteins are amphipathic in nature, comprising both hydrophilic and hydrophobic regions.
The intermediate portion of the phospholipid bilayer shows a hydrophobic character, while the rest of the part (protruded outwards) shows hydrophilic behaviour. Due to membrane fluidity, they move laterally within the biological cell membrane.
They are firmly attached to the cell membrane. Thus, the integral proteins are not easily separable and require ionic and non-ionic detergent treatment (like SDS and Triton X-100). SDS denatures the structure of proteins, while Triton X-100 does not alter the protein conformation.
Therefore, the solubilisation of integral proteins by the detergents helps us to study the composition of amino acids, molecular weight and other physicochemical properties. The integral proteins can be monotopic and polytopic, depending upon the number of alpha-helices.
- Monotopic integral proteins possess a single coiled alpha helix.
- Polytopic integral proteins possess combinations of coiled alpha-helices.
Not all the transmembrane proteins have alpha-helices; only a few have beta-barrel sheets. Glycophorin-A is the best example of an integral protein found in erythrocytes comprising 131 amino acid residues and primarily glycoproteins.
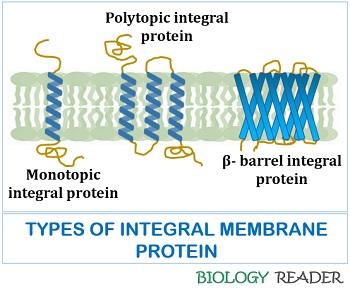
The transmembrane proteins have alpha-helices, which generally contain 21-26 hydrophobic amino acid residues. They undergo coiling to form alpha-helix and facilitate the spanning of the bilayer membrane.
Few transmembrane proteins consist of antiparallel arranged beta-strands, known as beta-barrels. Porin beta-barrels contain 16 stranded antiparallel beta-sheets with a highly hydrophilic interior and hydrophobic exterior.
Peripheral Proteins
They refer to the extrinsic proteins, which associate with the lipid bilayer through weak electrostatic and hydrogen bond interactions. Peripheral proteins are easily separable under the exposure of high pH and treatment with high salt concentration.
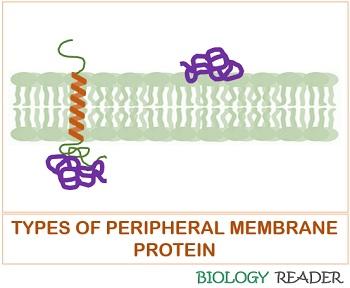
They are located directly on the polar heads of the phospholipid bilayer membrane or indirectly on the transmembrane channels. The peripheral proteins help in anchoring, cell support, and transmission of transmembrane signals.
It does not interface with the cell membrane’s hydrophobic core. Peripheral proteins are water-soluble and present higher in number than integral proteins. Extrinsic proteins are less mobile.
Lipid Anchored Proteins
They refer to the lipid-linked proteins, which binds covalently to the lipid membrane either through a fatty acid chain, prenyl group or often via an oligosaccharide complex. The peripheral proteins attach with the lipid membrane through glycosylphosphatidylinositol linkage to constitute “GPI- anchored proteins”.
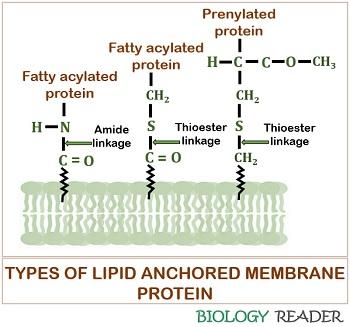
Lipid-linked proteins have the characteristic property that they are located on either side of the biological cell membrane. They belong to the class of “Proteolipids”.
Prenylated, fatty acylated, and glycosylphosphatidylinositol linked proteins are the three distinct kinds of lipid anchored proteins. Lipid-anchored proteins possess multiple lipid groups and serve as the hydrolytic enzymes, adhesion molecules, receptor proteins, etc.
Functions of Membrane Proteins
The lipid content of the cell membrane helps in forming a semipermeable barrier between the surrounding and protoplasm. Thus, transport proteins allow the influx and efflux of particular solutes.
Some transport proteins actively shuttle the various ions and molecules across the selective barrier by changing their conformation and hydrolyzing a high energy molecule ATP.
Membrane proteins also possess enzyme complexes, which carry out the sequential steps during different metabolic pathways. They also mediate signal transduction or signal-relaying by the specific binding between the chemical messengers with the binding site of the membrane proteins.
Glycoprotein-channels facilitate cell to cell recognition by specific binding between the receptors and ligands of the adjacent cells. The membrane proteins join at different gap junctions or show inter-cellular joining, which aids in cell-cell communication.
The cytoskeleton forms the interlinking protein filaments or microfilaments associated with the transport proteins, which promote cell shape and coordination between the cells doing extracellular and intracellular activities.